CROHN’S
EFFICACY
RESULTS
INDICATION
RINVOQ is indicated for the treatment of adults with moderately to severely active Crohn’s disease who have had an inadequate response or intolerance to one or more tumor necrosis factor (TNF) blockers.
Limitations of Use: RINVOQ is not recommended for use in combination with other Janus kinase (JAK) inhibitors, biological therapies for Crohn’s disease, or with potent immunosuppressants such as azathioprine and cyclosporine.
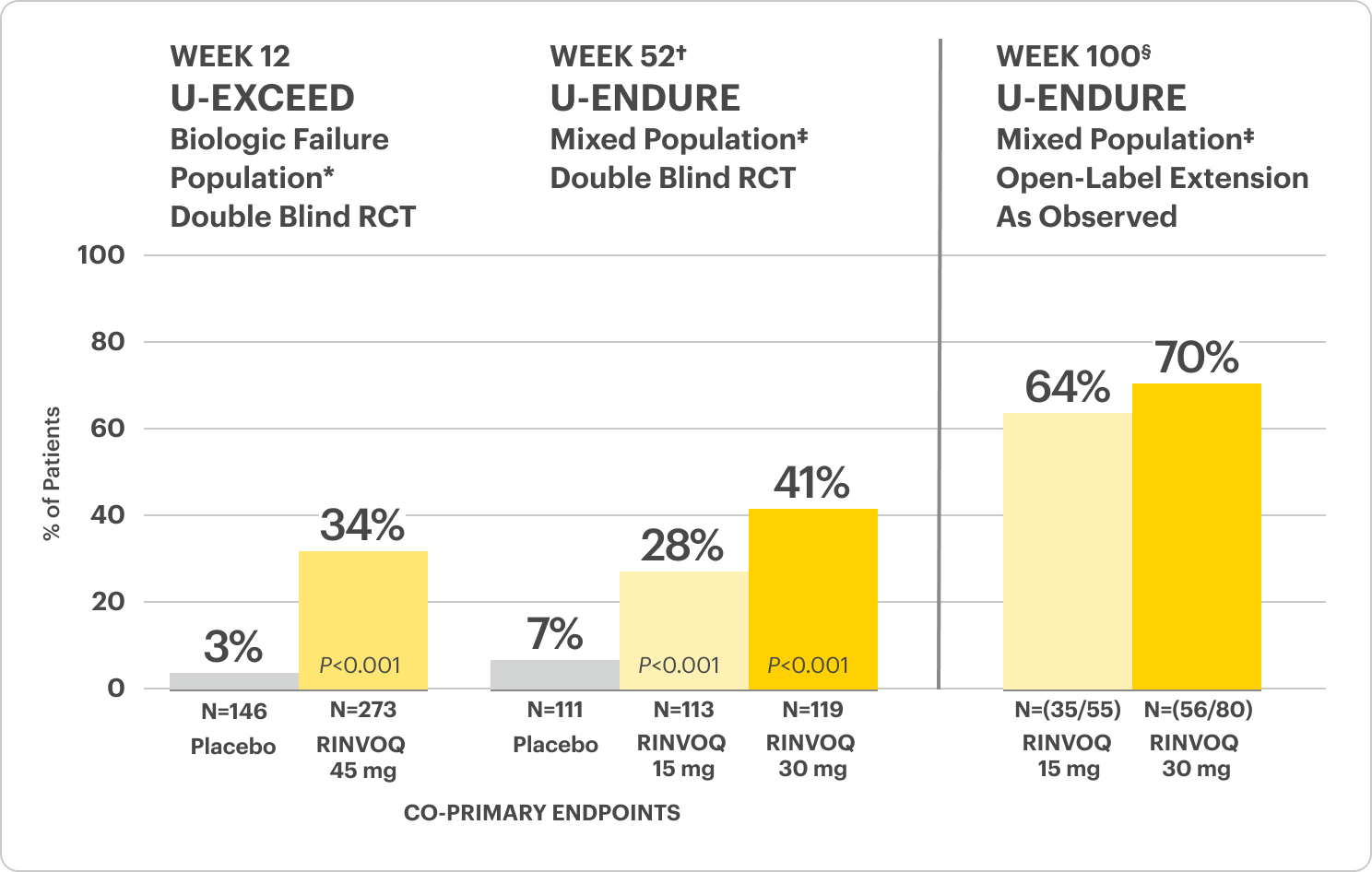
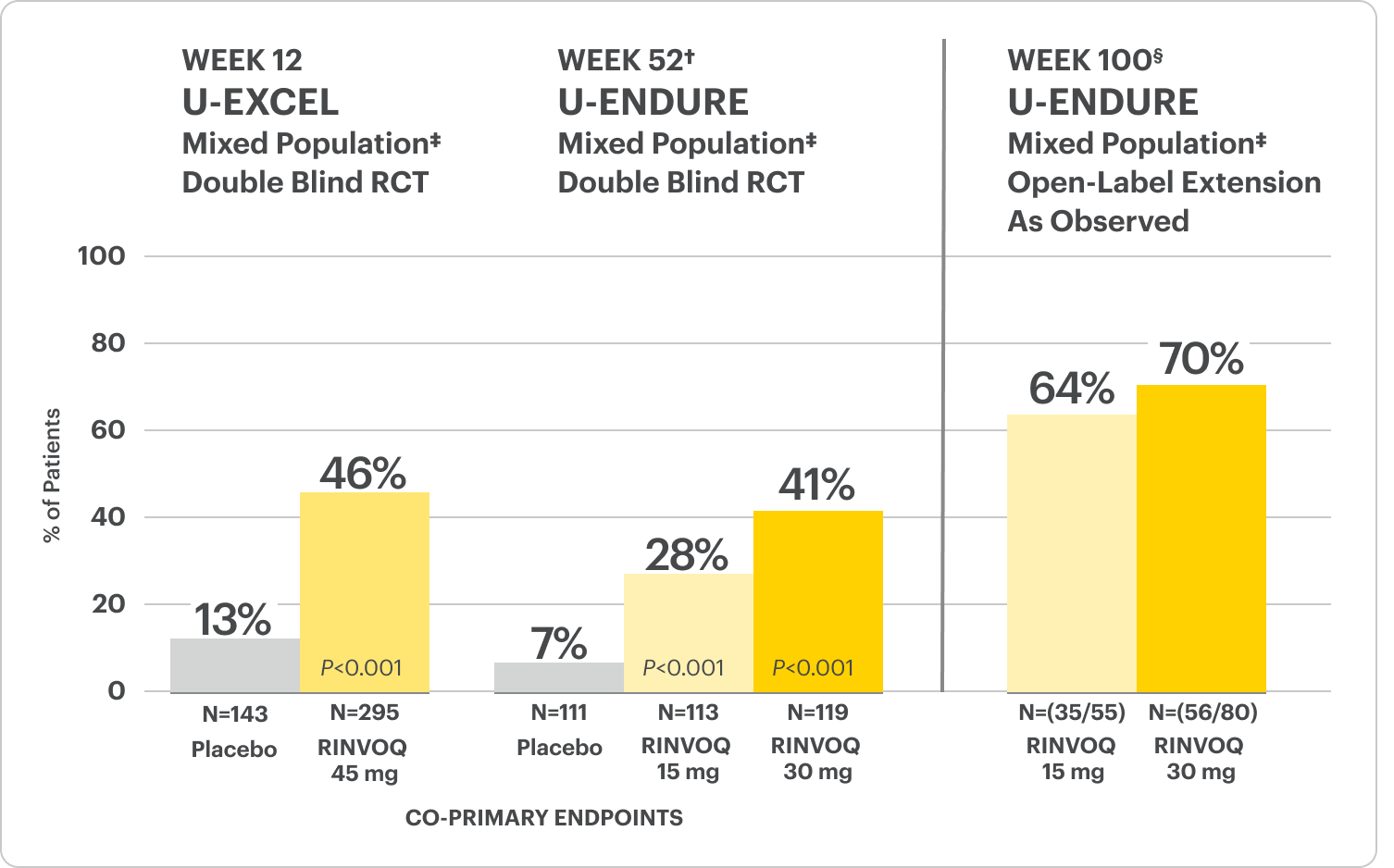
Significant Endoscopic Control1
Visible mucosal improvement with endoscopic response* and endoscopic remission† endpoints achieved at Weeks 12 and 52.
Durable Clinical Remission1
Clinical remission‡ achieved at Weeks 12 and 52.
Rapid Symptom Relief1
Clinical response§ achieved at Week 2.
*Endoscopic response was defined as a decrease in SES-CD >50% from baseline, or a decrease of at least 2 points for subjects with a baseline score of 4 and isolated ileal disease, based on central reading. The sections evaluated on endoscopy are the: rectum, sigmoid and left colon, transverse colon, right colon, and ileum (per SES-CD assessment).
†Endoscopic remission was defined as SES-CD ≤4 and no subscore >1 in any individual variable, as scored by a central reviewer.
‡Clinical remission was defined as CDAI <150 points.
§Clinical response was defined as a reduction of CDAI ≥100 points from baseline.
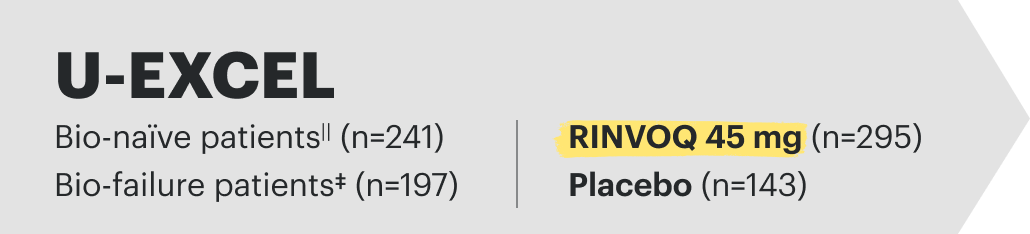
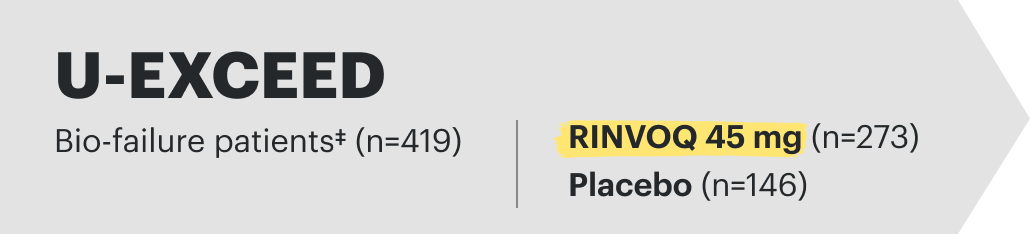
U-EXCEL Induction & U-EXCEED Induction Study Design Intro1: 12-week, double-blind, placebo-controlled, phase 3 induction studies that evaluated the efficacy and safety of RINVOQ in 857 adult patients (419 patients for U-EXCEED and 438 patients for U-EXCEL) with moderately to severely active Crohn's disease who demonstrated prior failure to biologic treatment (U-EXCEED) or prior failure to conventional and/or biologic treatment (U-EXCEL). Patients were randomized to receive RINVOQ 45 mg or placebo once daily for 12 weeks. The co-primary endpoints were the proportion of patients who achieved clinical remission (by CDAI) and endoscopic response (by SES-CD) at Week 12.
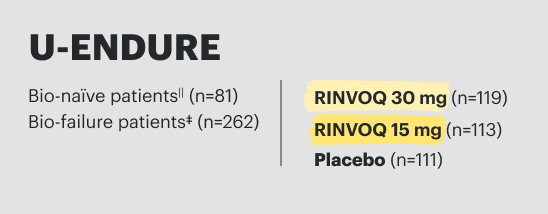
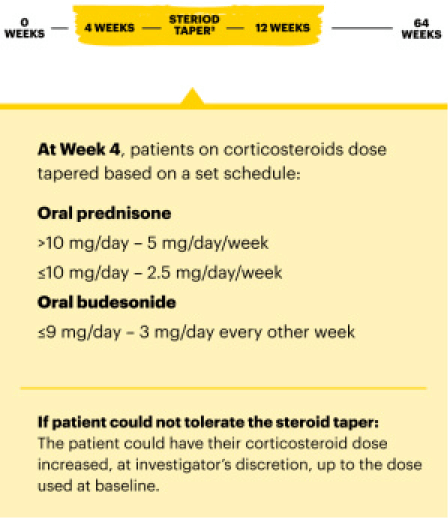
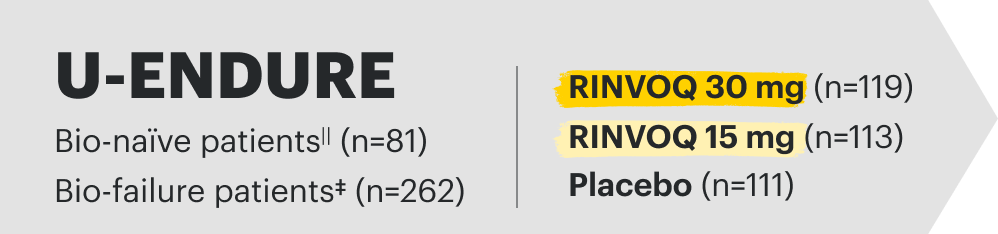
U-ENDURE Maintenance Study Design Intro1: 52-week, double-blind, placebo-controlled, phase 3 maintenance study of 343 adult patients with moderately to severely active Crohn’s disease who achieved clinical response (decrease in CDAI ≥100 points from baseline from RINVOQ induction) in the U-EXCEL and U-EXCEED studies. Patients were re-randomized to receive a maintenance regimen of either RINVOQ 15 mg, RINVOQ 30 mg, or placebo once daily for 52 weeks. The co-primary endpoints were the proportion of patients who achieved clinical remission (by CDAI) and endoscopic response (by SES-CD) at Week 52.
CDAI=Crohn's Disease Activity Index; IR=intolerance or inadequate response; SES-CD=Simple Endoscopic Score for Crohn's Disease; TNFi=tumor necrosis factor inhibitor.
Please see Important Safety Information, including BOXED WARNING on Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis, below.
Significant Endoscopic Control Visible Mucosal Improvement
Met at Weeks 12 and 52
Endoscopic Response Data Up to ~2 Years1,2
OLE LIMITATIONS: In an OLE, there is potential for enrichment of the long-term data in the remaining patient populations since patients who are unable to tolerate or do not respond to drug often drop out.
AO DISCLOSURE: In an as observed (AO) analysis, missing visit data was excluded from calculations from that visit, which may increase the percent of responders. All observed data was used regardless of premature discontinuation of study drug, or initiation of concomitant medications. The same patient may not have a response at each timepoint.
RINVOQ is indicated for TNFi-IR patients.
All P-values are RINVOQ treatment arms vs. placebo.
†Results at 52 weeks are among 343 patients who achieved clinical response|| after 12 weeks of treatment with RINVOQ in induction trials.
§Patients at Week 100 were on open-label treatment and knew the dose they were on. Data shown is an ongoing analysis and does not include all patients at Week 100 (Week 48 of open-label LTE).
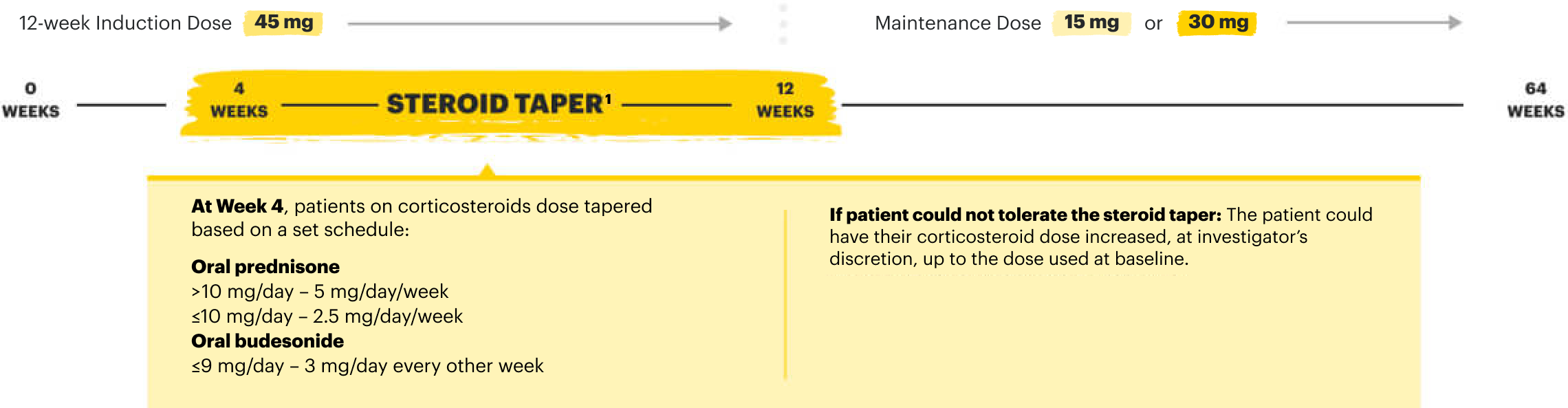
RECOMMENDED MAINTENANCE DOSING
A maintenance dose of 30 mg may be considered for patients with refractory, severe, or extensive disease. Discontinue RINVOQ if an adequate therapeutic response is not achieved with the 30 mg dose.1
*Prior biologic failure includes inadequate response, loss of response, or intolerance to one or more biologics.
‡The mixed population included patients who had inadequate response, loss of response, or intolerance to one or more biologics (biologic failure), as well as some patients who were not bio-exposed and some patients who were bio-exposed but did not have an inadequate response, loss of response, or intolerance to biologics (biologic naïve).
||Clinical response was defined as a reduction of CDAI ≥100 points from baseline.
OLE LIMITATIONS: In an OLE, there is potential for enrichment of the long-term data in the remaining patient populations since patients who are unable to tolerate or do not respond to drug often drop out.
AO DISCLOSURE: In an as observed (AO) analysis, missing visit data was excluded from calculations from that visit, which may increase the percent of responders. All observed data was used regardless of premature discontinuation of study drug, or initiation of concomitant medications. The same patient may not have a response at each timepoint.
RINVOQ is indicated for TNFi-IR patients.
All P-values are RINVOQ treatment arms vs. placebo.
†Results at 52 weeks are among 343 patients who achieved clinical response|| after 12 weeks of treatment with RINVOQ in induction trials.
§Patients at Week 100 were on open-label treatment and knew the dose they were on. Data shown are an ongoing analysis and do not include all patients at Week 100 (Week 48 of open-label LTE).
RECOMMENDED MAINTENANCE DOSING
A maintenance dose of 30 mg may be considered for patients with refractory, severe, or extensive disease. Discontinue RINVOQ if an adequate therapeutic response is not achieved with the 30 mg dose.1
*Prior biologic failure includes inadequate response, loss of response, or intolerance to one or more biologics.
‡The mixed population included patients who had inadequate response, loss of response, or intolerance to one or more biologics (biologic failure), as well as some patients who were not bio-exposed and some patients who were bio-exposed but did not have an inadequate response, loss of response, or intolerance to biologics (biologic naïve).
||Clinical response was defined as a reduction of CDAI ≥100 points from baseline.
Endoscopic Remission Data Up to ~2 Years1,2
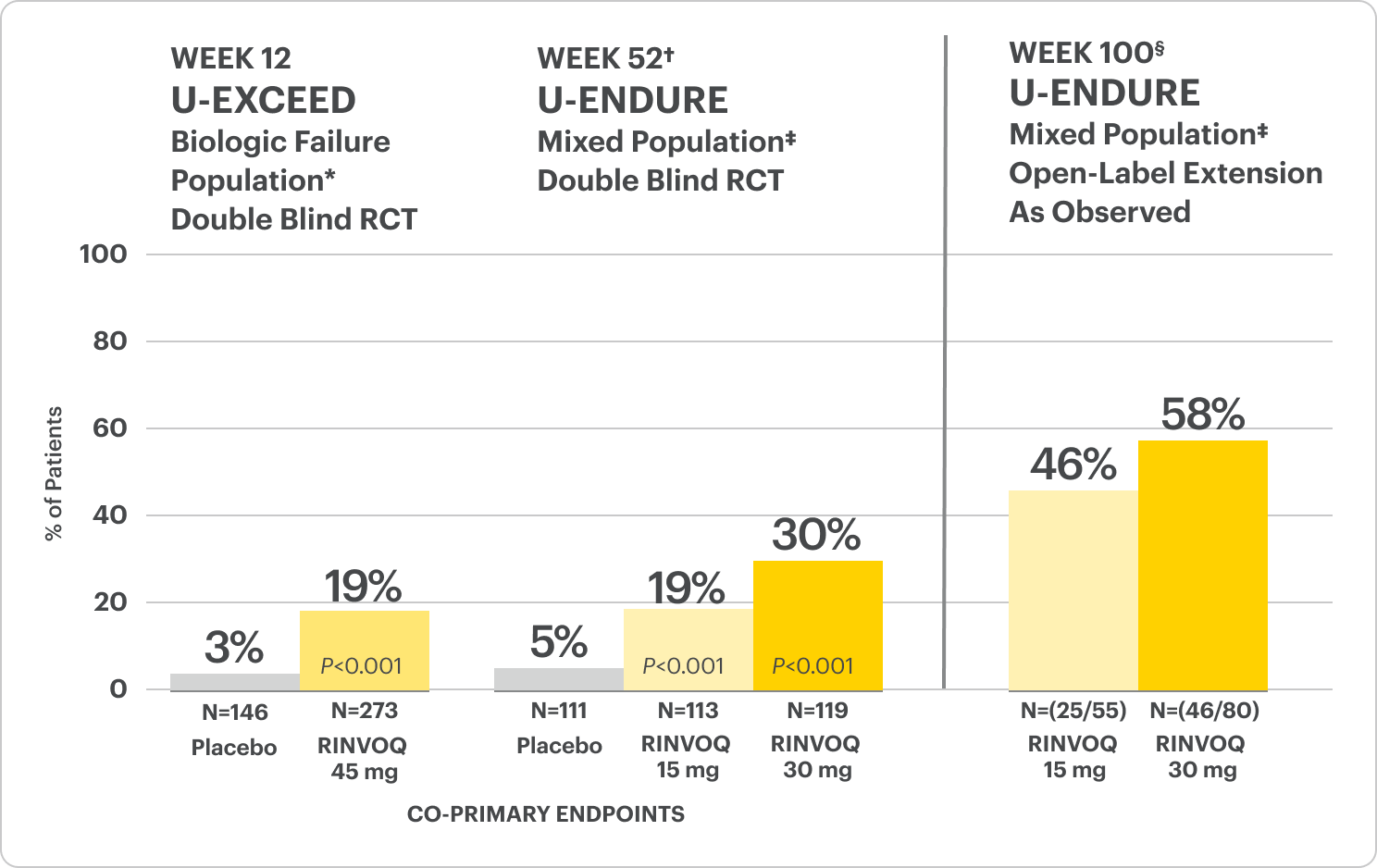
OLE LIMITATIONS: In an OLE, there is a potential for enrichment of the long-term data in the remaining patient populations since patients who are unable to tolerate or do not respond to drug often drop out.
AO DISCLOSURE: In an observed (AO) analysis, missing visit data was excluded from calculations from that visit, which may increase the percentage of responders. All observed data was used regardless of premature discontinuation of study drug, or initiation of concomitant medications. The same patient may not have a response at each timepoint.
RINVOQ is indicated for TNFi-IR patients.
All P-values are RINVOQ treatment arms vs. placebo.
†Results at 52 weeks are among 343 patients who achieved clinical response|| after 12 weeks of treatment with RINVOQ in induction trials.
§Patients at Week 100 were on open-label treatment and knew the dose they were on. Data shown is an ongoing analysis and does not include all patients at Week 100 (Week 48 of open-label LTE).
RECOMMENDED MAINTENANCE DOSING
A maintenance dose of 30 mg may be considered for patients with refractory, severe, or extensive disease. Discontinue RINVOQ if an adequate therapeutic response is not achieved with the 30 mg dose.1
*Prior biologic failure includes inadequate response, loss of response, or intolerance to one or more biologics.
†The mixed population included patients who had inadequate response, loss of response, or intolerance to one or more biologics (biologic failure), as well as some patients who were not bio-exposed and some patients who were bio-exposed but did not have an inadequate response, loss of response, or intolerance to biologics (biologic naïve).
||Clinical response was defined as a reduction of CDAI ≥100 points from baseline.
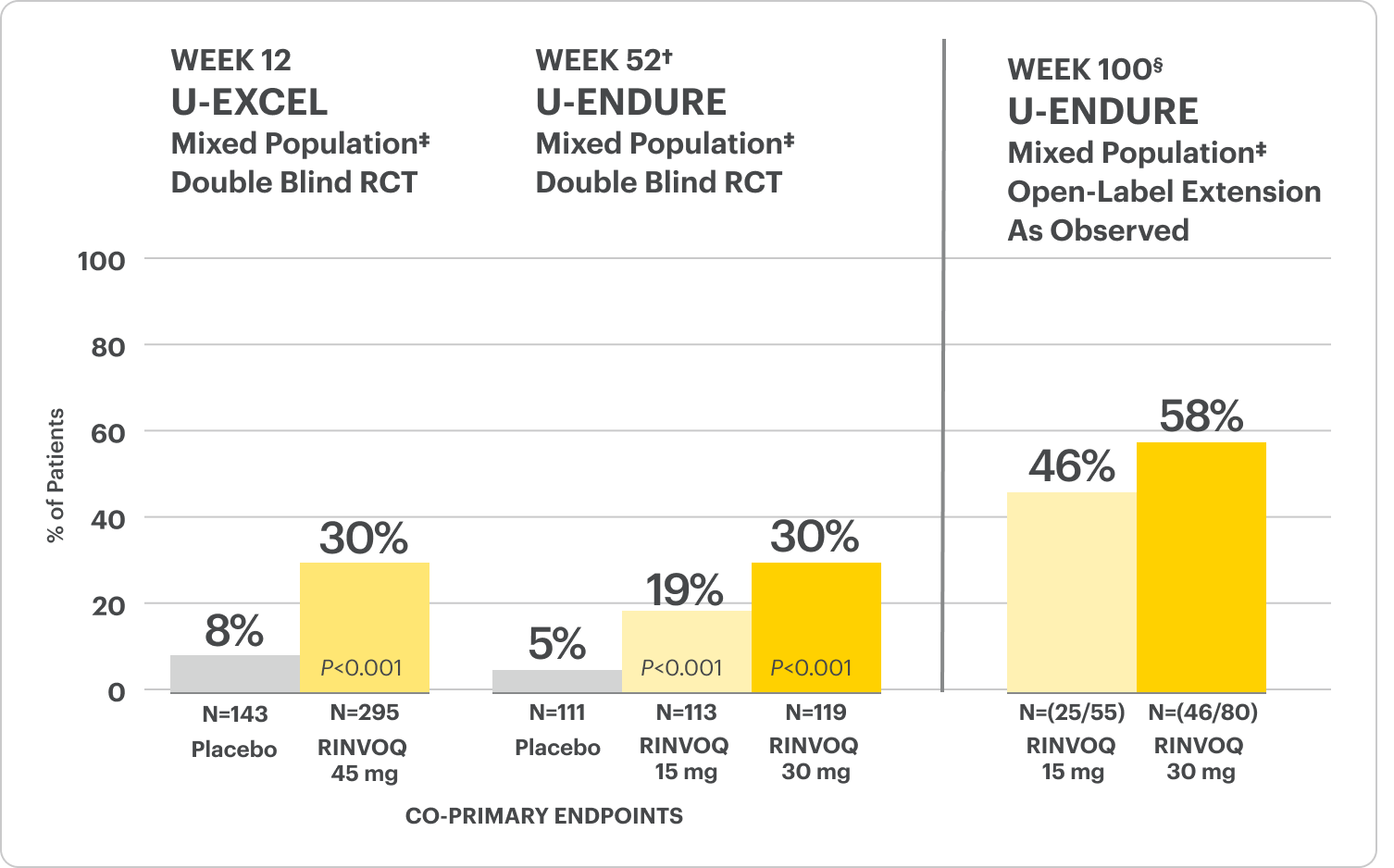
OLE LIMITATIONS: In an OLE, there is a potential for enrichment of the long-term data in the remaining patient populations since patients who are unable to tolerate or do not respond to drug often drop out.
AO DISCLOSURE: In an observed (AO) analysis, missing visit data was excluded from calculations from that visit, which may increase the percentage of responders. All observed data was used regardless of premature discontinuation of study drug, or initiation of concomitant medications. The same patient may not have a response at each timepoint.
RINVOQ is indicated for TNFi-IR patients.
All P-values are RINVOQ treatment arms vs. placebo.
†Results at 52 weeks are among 343 patients who achieved clinical response|| after 12 weeks of treatment with RINVOQ in induction trials.
§Patients at Week 100 were on open-label treatment and knew the dose they were on. Data shown is an ongoing analysis and does not include all patients at Week 100 (Week 48 of open-label LTE).
RECOMMENDED MAINTENANCE DOSING
A maintenance dose of 30 mg may be considered for patients with refractory, severe, or extensive disease. Discontinue RINVOQ if an adequate therapeutic response is not achieved with the 30 mg dose.1
*Prior biologic failure includes inadequate response, loss of response, or intolerance to one or more biologics.
‡The mixed population included patients who had inadequate response, loss of response, or intolerance to one or more biologics (biologic failure), as well as some patients who were not bio-exposed and some patients who were bio-exposed but did not have an inadequate response, loss of response, or intolerance to biologics (biologic naïve).
||Clinical response was defined as a reduction of CDAI ≥100 points from baseline.
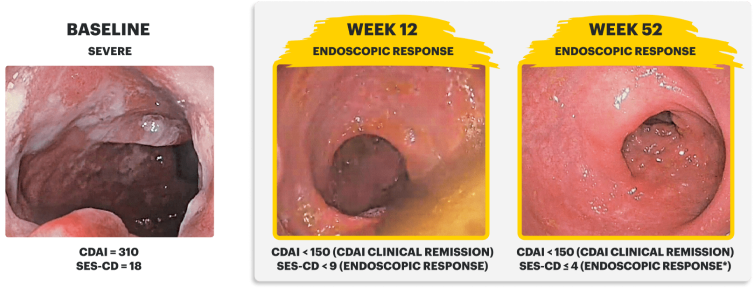
Real Patient Case: Clinical Trial3
Sex: Female | Age: 40s | Disease Duration: 2.9 Years | Severe Crohn's, Prior TNFi-IR3
Patient received RINVOQ 45 mg QD (induction) and RINVOQ 30 mg QD (maintenance)*
RECOMMENDED MAINTENANCE DOSING
A maintenance dose of 30 mg may be considered for patients with refractory, severe, or extensive disease. Discontinue RINVOQ if an adequate therapeutic response is not achieved with the 30 mg dose.1
IMAGES ARE FROM A CLINICAL TRIAL PATIENT TREATED WITH RINVOQ. INDIVIDUAL RESULTS MAY VARY.
CDAI clinical remission is defined as CDAI score <150.
Endoscopic response was defined as a decrease in SES-CD >50% from baseline, or a decrease of at least 2 points for subjects with a baseline score of 4 and isolated ileal disease, based on central reading. The sections evaluated on endoscopy are the: rectum, sigmoid and left colon, transverse colon, right colon, and ileum (per SES-CD assessment).
Endoscopic remission was defined as SES-CD ≤4 and no subscore >1 in any individual variable, as scored by a central reviewer.
*Patient did not meet endoscopic remission due to a subscore >1 in an individual variable of SES-CD measure.
QD=once daily.
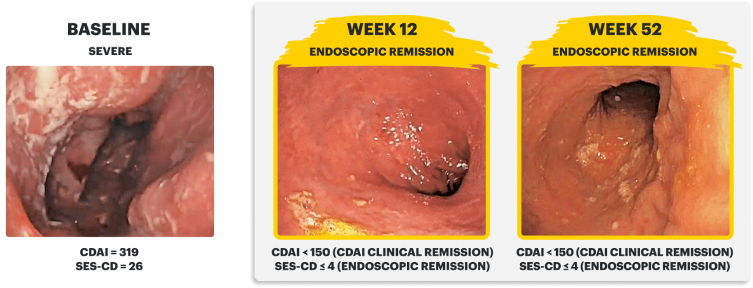
Sex: Male | Age: 30s | Disease Duration: 28.8 Years | Severe Crohn's, Prior TNFi-IR3
Patient received RINVOQ 45 mg QD (induction) and RINVOQ 15 mg QD (maintenance)
In the U-EXCEED induction study, 19% (N=273) on RINVOQ 45 mg versus 3% (N=146) on placebo achieved endoscopic remission at Week 12 (P<0.001).1
In the U-ENDURE maintenance study, among patients who achieved clinical response* after 12 weeks of RINVOQ 45 mg once-daily induction treatment, 16% (N=113) on RINVOQ 15 mg versus 4% (N=111) on placebo achieved clinical and endoscopic remission at Week 52 (P<0.001). Among bio-failure patients, 13% (N=85) on RINVOQ 15 mg versus 2% (N=87) on placebo had clinical and endoscopic remission at Week 52.1
All P-values are RINVOQ treatment arms vs. placebo.
IMAGES ARE FROM A CLINICAL TRIAL PATIENT TREATED WITH RINVOQ. INDIVIDUAL RESULTS MAY VARY.
CDAI clinical remission is defined as CDAI score <150.3
Endoscopic response was defined as a decrease in SES-CD >50% from baseline, or a decrease of at least 2 points for subjects with a baseline score of 4 and isolated ileal disease, based on central reading. The sections evaluated on endoscopy are the: rectum, sigmoid and left colon, transverse colon, right colon, and ileum (per SES-CD assessment).3
Endoscopic remission was defined as SES-CD ≤4 and no subscore >1 in any individual variable, as scored by a central reviewer.3
*Clinical response was defined as a reduction of CDAI ≥100 points from baseline.
QD=once daily.
Safety Considerations
Serious Infections: RINVOQ-treated patients are at increased risk of serious bacterial (including tuberculosis [TB]), fungal, viral, and opportunistic infections leading to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants, such as methotrexate or corticosteroids.
Mortality: A higher rate of all-cause mortality, including sudden cardiovascular (CV) death, was observed with a Janus kinase inhibitor (JAKi) in a study comparing another JAKi with tumor necrosis factor (TNF) blockers in rheumatoid arthritis (RA) patients ≥50 years with ≥1 CV risk factor.
Malignancies: Malignancies have occurred in RINVOQ-treated patients. A higher rate of lymphomas and lung cancer (in current or past smokers) was observed with another JAKi when compared with TNF blockers in RA patients.
Major Adverse Cardiovascular Events: A higher rate of CV death, myocardial infarction, and stroke was observed with a JAKi in a study comparing another JAKi with TNF blockers in RA patients ≥50 years with ≥1 CV risk factor. History of smoking increases risk.
Thrombosis: Deep venous thrombosis, pulmonary embolism, and arterial thrombosis have occurred in patients treated with JAK inhibitors used to treat inflammatory conditions. A higher rate of thrombosis was observed with another JAKi when compared with TNF blockers in RA patients.
Hypersensitivity: RINVOQ is contraindicated in patients with hypersensitivity to RINVOQ or its excipients.
Other Serious Adverse Reactions: Hypersensitivity Reactions, Gastrointestinal Perforations, Laboratory Abnormalities, and Embryo-Fetal Toxicity.
U-EXCEL Induction & U-EXCEED Induction Study Design Intro1: 12-week, double-blind, placebo-controlled, phase 3 induction studies that evaluated the efficacy and safety of RINVOQ in 857 adult patients (419 patients for U-EXCEED and 438 patients for U-EXCEL) with moderately to severely active Crohn's disease who demonstrated prior failure to biologic treatment (U-EXCEED) or prior failure to conventional and/or biologic treatment (U-EXCEL). Patients were randomized to receive RINVOQ 45 mg or placebo once daily for 12 weeks. The co-primary endpoints were the proportion of patients who achieved clinical remission (by CDAI) and endoscopic response (by SES-CD) at Week 12.
U-ENDURE Maintenance Study Design Intro1: 52-week, double-blind, placebo-controlled, phase 3 maintenance study of 343 adult patients with moderately to severely active Crohn’s disease who achieved clinical response (decrease in CDAI ≥100 points from baseline from RINVOQ induction) in the U-EXCEL and U-EXCEED studies. Patients were re-randomized to receive a maintenance regimen of either RINVOQ 15 mg, RINVOQ 30 mg, or placebo once daily for 52 weeks. The co-primary endpoints were the proportion of patients who achieved clinical remission (by CDAI) and endoscopic response (by SES-CD) at Week 52.
Durable Clinical Remission
Met at Weeks 12 and 52
Clinical Remission Data Up to ~2 Years1,2
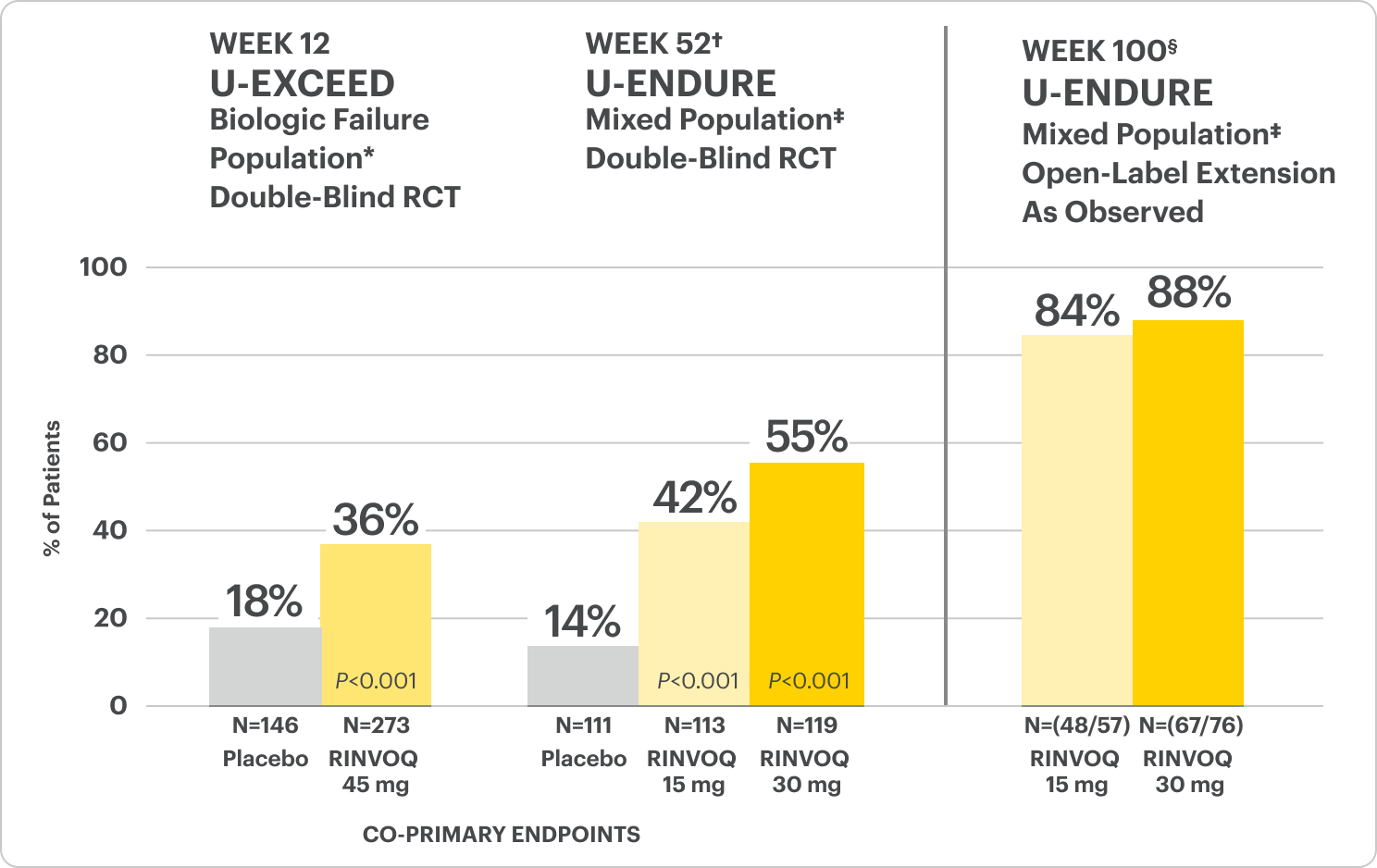
OLE LIMITATIONS: In an OLE, there is potential for enrichment of the long-term data in the remaining patient populations since patients who are unable to tolerate or do not respond to drug often drop out.
AO DISCLOSURE: In an as observed (AO) analysis, missing visit data was excluded from calculations from that visit, which may increase the percent of responders. All observed data was used regardless of premature discontinuation of study drug, or initiation of concomitant medications. The same patient may not have a response at each timepoint.
RINVOQ is indicated for TNFi-IR patients.
All P-values are RINVOQ treatment arms vs. placebo.
†Results at 52 weeks are among 343 patients who achieved clinical response|| after 12 weeks of treatment with RINVOQ in induction trials.
§Patients at Week 100 were on open-label treatment and knew the dose they were on. Data shown is an ongoing analysis and do not include all patients at Week 100 (Week 48 of open-label LTE).
RECOMMENDED MAINTENANCE DOSING
A maintenance dose of 30 mg may be considered for patients with refractory, severe, or extensive disease. Discontinue RINVOQ if an adequate therapeutic response is not achieved with the 30 mg dose.1
*Prior biologic failure includes inadequate response, loss of response, or intolerance to one or more biologics.
‡The mixed population included patients who had inadequate response, loss of response, or intolerance to one or more biologics (biologic failure), as well as some patients who were not bio-exposed and some patients who were bio-exposed but did not have an inadequate response, loss of response, or intolerance to biologics (biologic naïve).
||Clinical response was defined as a reduction of CDAI ≥100 points from baseline.
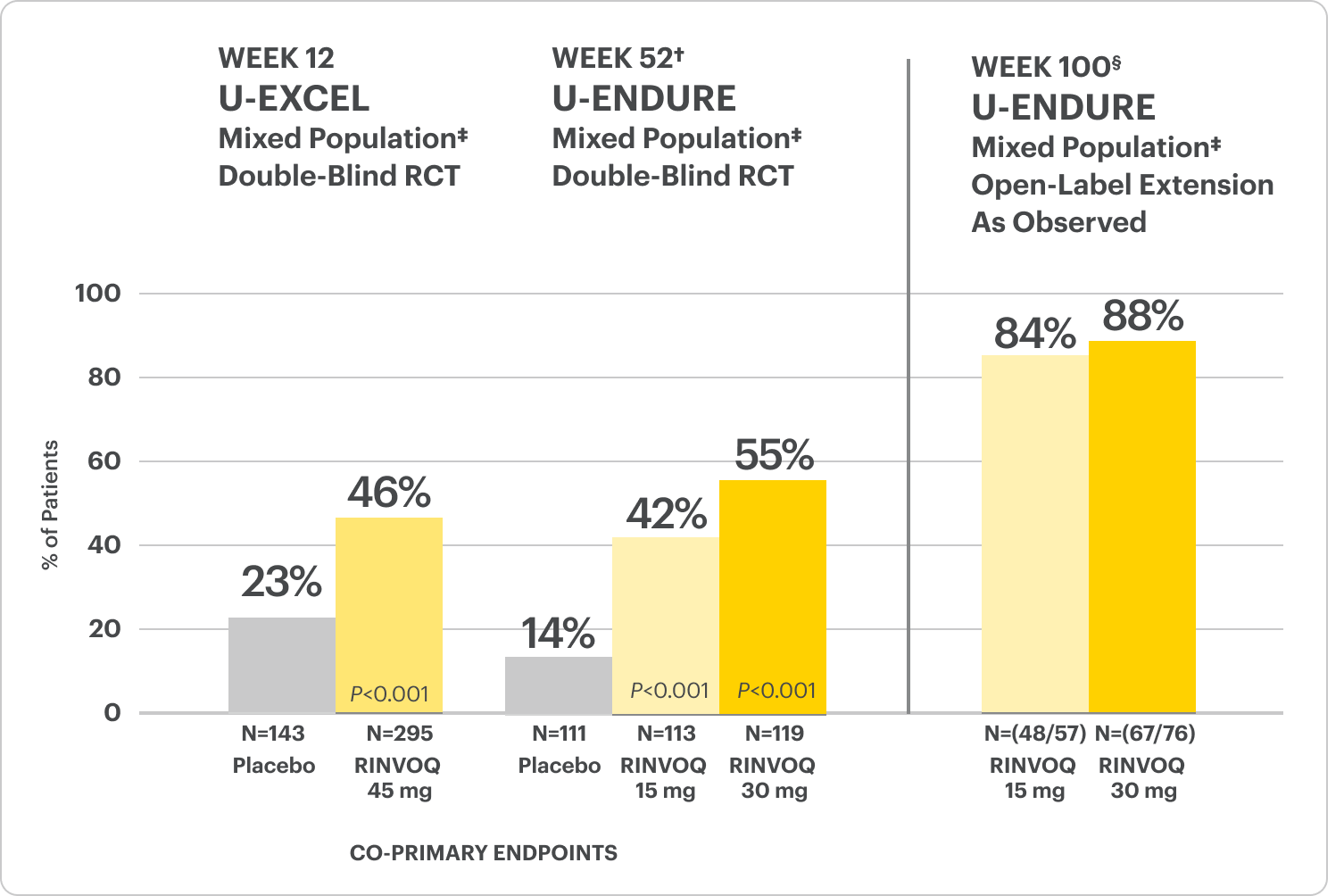
OLE LIMITATIONS: In an OLE, there is a potential for enrichment of the long-term data in the remaining patient populations since patients who are unable to tolerate or do not respond to drug often drop out.
AO DISCLOSURE: In an observed (AO) analysis, missing visit data was excluded from calculations from that visit, which may increase the percentage of responders. All observed data was used regardless of premature discontinuation of study drug, or initiation of concomitant medications. The same patient may not have a response at each timepoint.
RINVOQ is indicated for TNFi-IR patients.
All P-values are RINVOQ treatment arms vs. placebo.
†Results at 52 weeks are among 343 patients who achieved clinical response|| after 12 weeks of treatment with RINVOQ in induction trials.
§Patients at week 100 were on open-label treatment and knew the dose they were on. Data shown is an ongoing analysis and do not include all patients at week 100 (week 48 of open-label LTE).
RECOMMENDED MAINTENANCE DOSING
A maintenance dose of 30 mg may be considered for patients with refractory, severe, or extensive disease. Discontinue RINVOQ if an adequate therapeutic response is not achieved with the 30 mg dose.1
*Prior biologic failure includes inadequate response, loss of response, or intolerance to one or more biologics.
‡The mixed population included patients who had inadequate response, loss of response, or intolerance to one or more biologics (biologic failure), as well as some patients who were not bio-exposed and some patients who were bio-exposed but did not have an inadequate response, loss of response, or intolerance to biologics (biologic naïve).
||Clinical response was defined as a reduction of CDAI ≥100 points from baseline.
Rapid Symptom Relief at Week 2
Clinical Response (CDAI CR-100) at Weeks 2 and 121,4

RINVOQ is indicated for TNFi-IR patients.
All P-values are RINVOQ treatment arms vs. placebo.
DATA LIMITATIONS: Data labeled as a ranked secondary endpoint were multiplicity controlled for comparisons. All other comparisons were not adjusted for multiplicity; therefore, statistical significance has not been established.
*Prior biologic failure includes inadequate response, loss of response, or intolerance to one or more biologics.
†The mixed population included patients who had inadequate response, loss of response, or intolerance to one or more biologics (biologic failure), as well as some patients who were not bio-exposed and some patients who were bio-exposed but did not have an inadequate response, loss of response, or intolerance to biologics (biologic naïve).
Clinical Response (CDAI CR-100) at Weeks 2 and 121,4

RINVOQ is indicated for TNFi-IR patients.
All P-values are RINVOQ treatment arms vs. placebo.
DATA LIMITATIONS: Data labeled as a ranked secondary endpoint were multiplicity controlled for comparisons. All other comparisons were not adjusted for multiplicity; therefore, statistical significance has not been established.
*Prior biologic failure includes inadequate response, loss of response, or intolerance to one or more biologics.
†The mixed population included patients who had inadequate response, loss of response, or intolerance to one or more biologics (biologic failure), as well as some patients who were not bio-exposed and some patients who were bio-exposed but did not have an inadequate response, loss of response, or intolerance to biologics (biologic naïve).

Interested in the safety data for RINVOQ?
See RINVOQ’s safety data across clinical trials




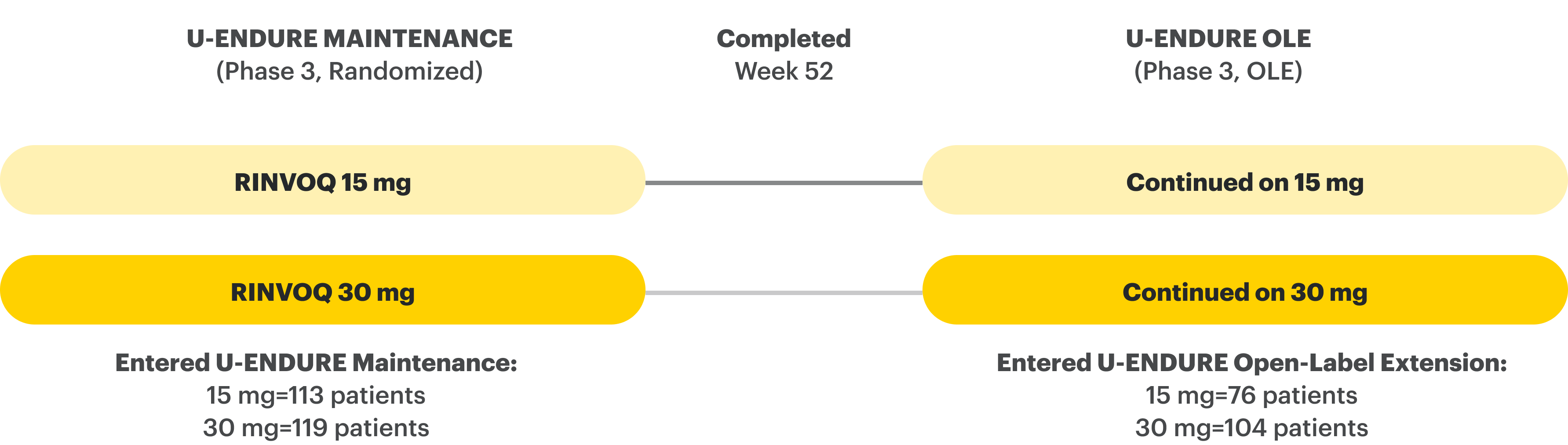









![52-week maintenance trial (randomized double-blind, placebo-controlled trial that evaluated patients who achieved clinical response after 12 weeks of treatment with RINVOQ 45 mg once-daily in induction trials [N=343]).](/content/dam/rinvoqhcpivy/images/gastroenterology/cd/maintenance-trials-mobile.png)