Images not reflective of actual pill size.
ONE PILL,
ONCE DAILY1
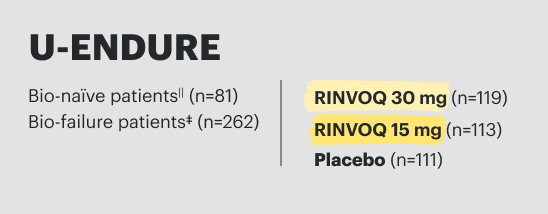
RINVOQ Dosing
A Once-Daily Pill That Comes in 3 Dosage Strengths1
1 Strength for Induction and 2 Strengths for Maintenance
1 Strength for Induction and 2 Strengths for Maintenance
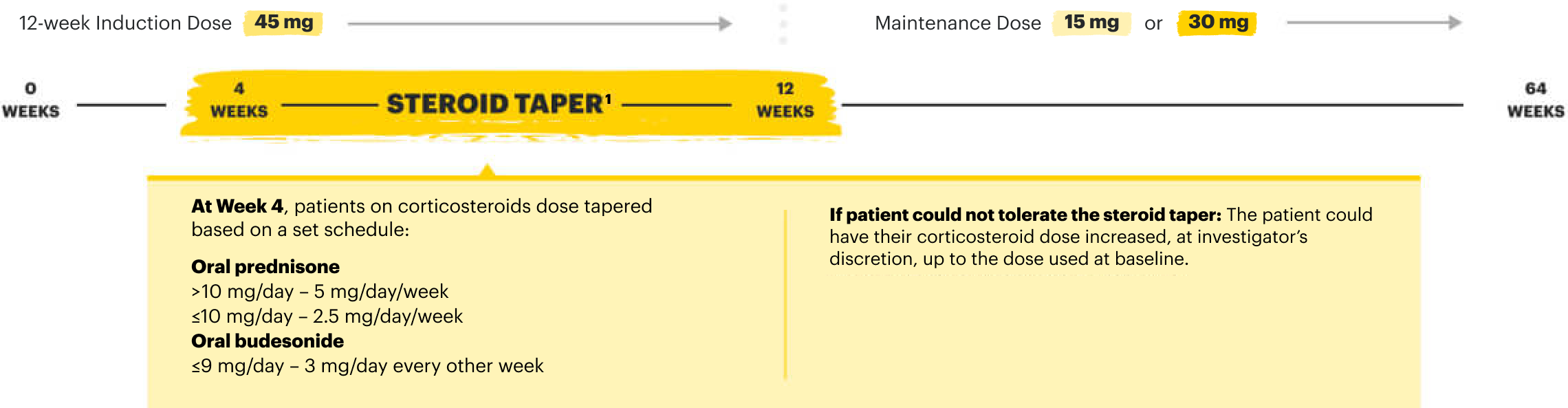
Induction Dose

RECOMMENDED INDUCTION DOSE
CD: 45 mg once daily for 12 weeks
UC: 45 mg once daily for 8 weeks
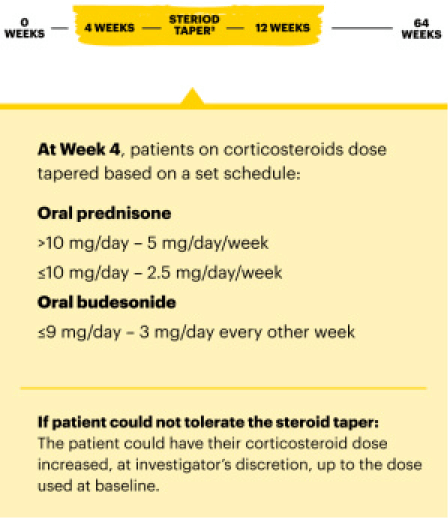
Maintenance Dose

Images not reflective of actual pill size.
RECOMMENDED MAINTENANCE DOSE
15 mg once daily for CD and UC
- 30 mg once daily may be considered for patients with refractory, severe, or extensive disease
- Discontinue RINVOQ if an adequate therapeutic response is not achieved with the 30 mg dose
- Use the lowest effective dosage needed to maintain response
CD=Crohn’s disease; NDC=National Drug Code; UC=ulcerative colitis.
ADMINISTRATION
- Instruct patients to take 1 pill, once daily1
- Ensure pill is taken whole. Do not split, crush, or chew1
- RINVOQ can be taken with or without food. Advise patients to avoid food or drink containing grapefruit during treatment with RINVOQ1
MEDICATION RESIDUE IN STOOL
- Instruct patients to notify their healthcare provider if they repeatedly notice intact RINVOQ tablet or fragments in stool or ostomy output1
Limitations of Use for CD and UC: RINVOQ is not recommended for use in combination with other JAK inhibitors, biological therapies for CD or UC, or with potent immunosuppressants such as azathioprine and cyclosporine.1
Please see Important Safety Information, including BOXED WARNING on Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis, below.
Lab Monitoring & Dosing Considerations
Perform lab testing for1:
| Laboratory Parameter | Check Lab Values | Treatment should NOT be INITIATED or CONTINUED if: | |
|---|---|---|---|
CBC Differential
|
Neutrophils | At baseline and periodically thereafter according to routine patient management | <1000 cells/mm3* |
| Lymphocytes | <500 cells/mm3* | ||
| Hemoglobin | <8 g/dL* | ||
| Liver enzymes | Elevated liver enzymes and suspected drug-induced injury | ||
| Lipidsa | At 12 weeks and thereafter according to clinical guidelines | ||
INTERRUPT IF PATIENT DEVELOPS A SERIOUS OR OPPORTUNISTIC INFECTION.
*Treatment can be initiated or restarted after levels return above specified values, drug-induced liver injury diagnosis is excluded, or infection is controlled.1
aLipids include total cholesterol, LDL cholesterol, and HDL cholesterol.
CBC=complete blood count; HDL=high-density lipoprotein; LDL=low-density lipoprotein
Lab abnormalities across doses from the placebo-controlled induction and maintenance studies1-3
<<Swipe table to see more
| CD INDUCTION (WEEK 12) | CD MAINTENANCE (WEEK 52) | UC INDUCTION (WEEK 8) | UC MAINTENANCE (WEEK 52) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LAB ABNORMALITY | Placebo (N=347) | RINVOQ 45 mg (N=674) | Placebo (N=223) | RINVOQ 15 mg (N=221) | RINVOQ 30 mg (N=229) | Placebo (N=378) | RINVOQ 45 mg (N=719) | Placebo (N=245) | RINVOQ 15 mg (N=250) | RINVOQ 30 mg (N=251) | ||||
| Liver Transaminases | ALT ≥3 x ULN | 2.9% | 2.1% | 1.8% | 0.9% | 2.6% | 0% | 1.5% | 0.8% | 2% | 4% | |||
| ALT ≥5 x ULN | 0% | 0.4% | 0.5% | 0% | 0% | 0% | 0.4% | 0.4% | 0.4% | 0.8% | ||||
| AST ≥3 x ULN | 0.9% | 1.5% | 1.4% | 0.9% | 0.9% | 0.3% | 1.5% | 0.4% | 1.6% | 2% | ||||
| Creatine Phosphokinase Elevations | >5 x ULN | 0.6% | 2.4% | 0.9% | 1.4% | 4.8% | 0.3% | 2.2% | 1.2% | 4.0% | 6.4% | |||
| Absolute Neutrophil Count | <1000 cells/mm3 | 0% | 0.9% | 0% | 0.9% | 2.2% | 0% | 2.8% | 0.8% | 0.8% | 2.4% | |||
| Absolute Lymphocyte Count | <500 cells/mm3 | 2.0% | 2.2% | 1.8% | 3.7% | 3.9% | 0.8% | 2% | 0.8% | 1.6% | 0.8% | |||
| Hemoglobin | <8 g/dL | 1.4% | 2.7% | 2.3% | 1.4% | 3.5% | 2.1% | 0.3% | 1.2% | 0.4% | 0.4% | |||
Lipid elevations: RINVOQ treatment was associated with increases in lipid parameters including total cholesterol, LDL cholesterol, and HDL cholesterol in placebo-controlled induction and maintenance studies.1





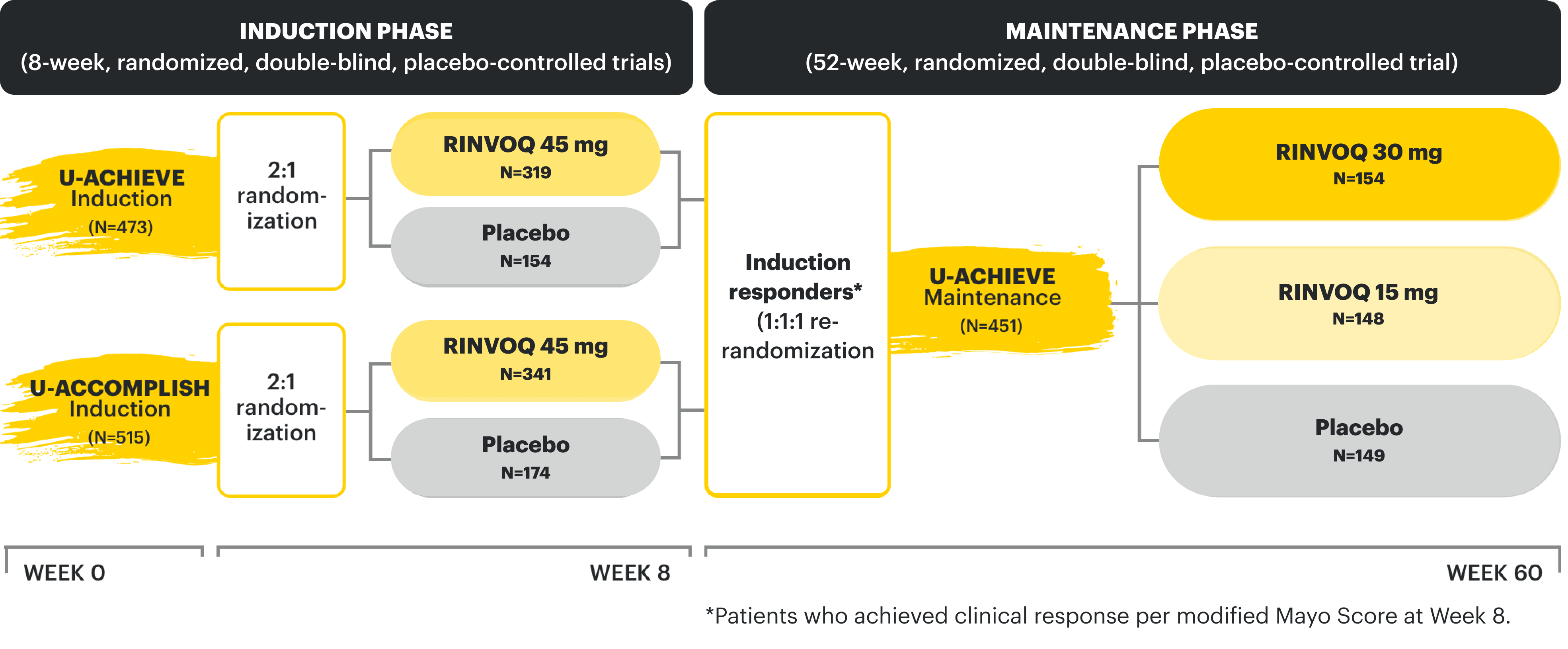
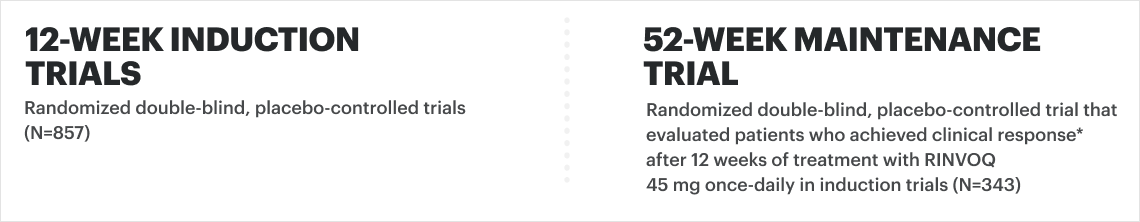

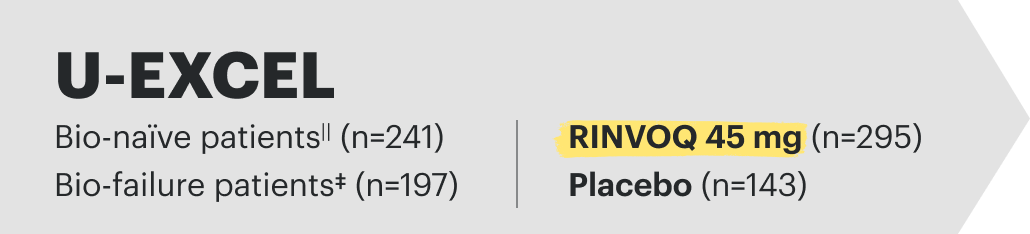

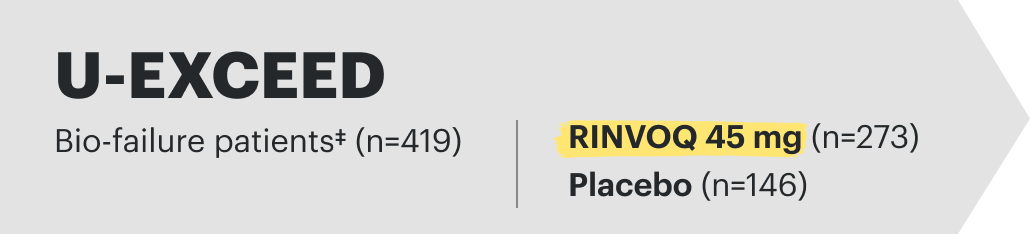


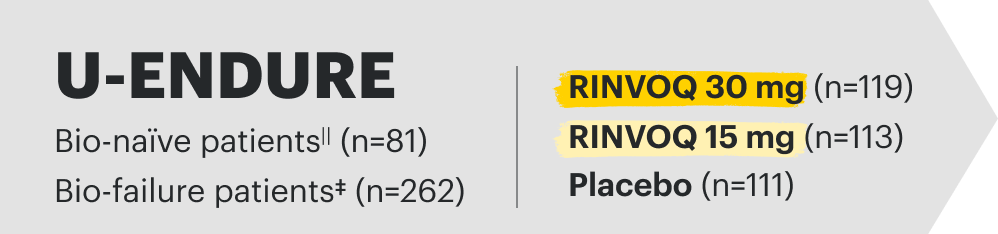





![52-week maintenance trial (randomized double-blind, placebo-controlled trial that evaluated patients who achieved clinical response after 12 weeks of treatment with RINVOQ 45 mg once-daily in induction trials [N=343]).](/content/dam/rinvoqhcpivy/images/gastroenterology/cd/maintenance-trials-mobile.png)