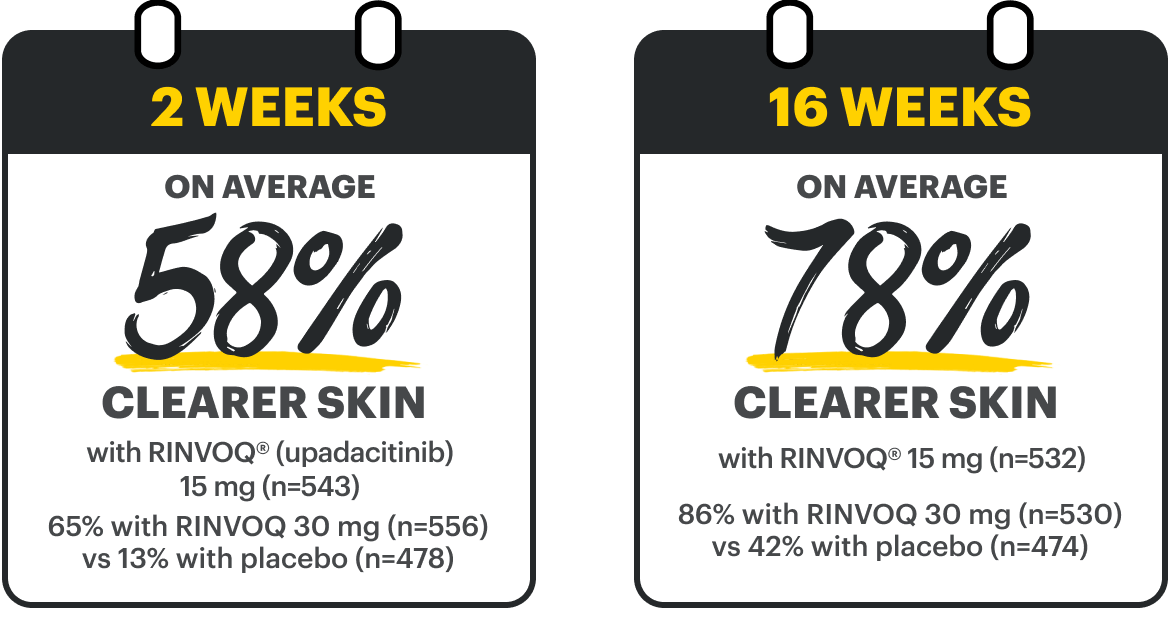
HIGHER-LEVEL DISEASE CONTROL*
IN BOTH ITCH & SKIN2,3
*Defined by simultaneous achievement of WP-NRS 0/1 & EASI 90; higher than WP-NRS ≥4 improvement and EASI 75. Itch & skin are clinical features of AD; other outcome measures may exist.
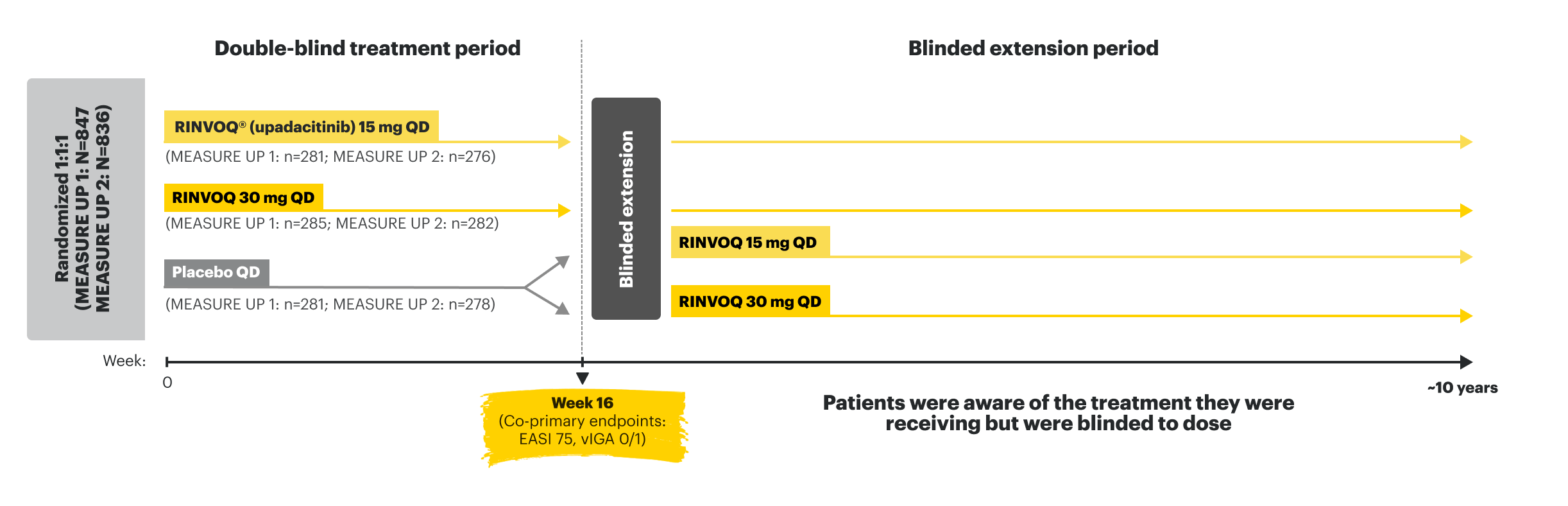
Pivotal Trial Endpoints in MEASURE UP 1 and MEASURE UP 21,4
Double-blind, placebo-controlled; NRI-C; ITT
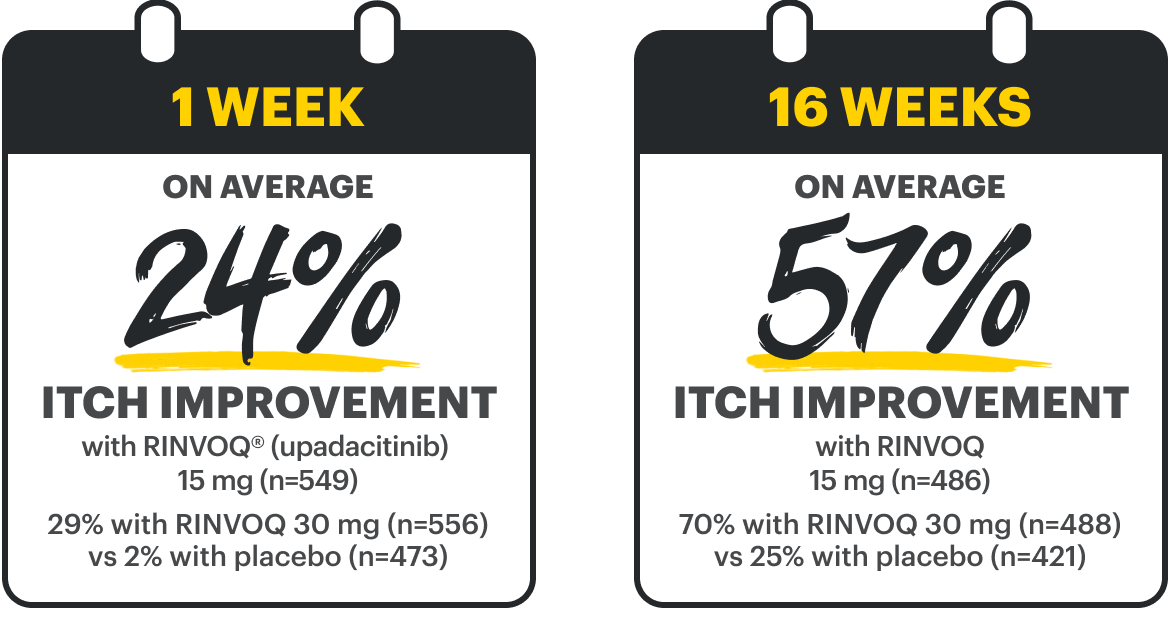
WP-NRS ≥4 improvement at Week 16
Ranked secondary endpoint
MEASURE UP 1:
RINVOQ 15 mg 52%† (n=274)
RINVOQ 30 mg 60%† (n=280)
Placebo 12% (n=272)
MEASURE UP 2:
RINVOQ 15 mg 42%† (n=270)
RINVOQ 30 mg 60%† (n=280)
Placebo 9% (n=274)
EASI 75 at Week 16
Co-primary endpoint
MEASURE UP 1:
RINVOQ 15 mg 70%†(n=281)
RINVOQ 30 mg 80%† (n=285)
Placebo 16% (n=281)
MEASURE UP 2:
RINVOQ 15 mg 60%†(n=276)
RINVOQ 30 mg 73%† (n=282)
Placebo 13% (n=278)
vIGA 0/1 at Week 16
Co-primary endpoint
MEASURE UP 1:
RINVOQ 15 mg 48%† (n=281)
RINVOQ 30 mg 62%†(n=285)
Placebo 8% (n=281)
MEASURE UP 2:
RINVOQ 15 mg 39%†(n=276)
RINVOQ 30 mg 52%† (n=282)
Placebo 5% (n=278)
†p≤0.001; RINVOQ vs placebo.
RECOMMENDED DOSAGE IN AD1:
- 30 mg is not an approved starting dose
- For pediatric patients ≥12 years (40+ kg) and adults <65 years old: Initiate with 15 mg once daily. If an adequate response is not achieved, consider increasing dosage to 30 mg once daily. Discontinue if an adequate response is not achieved with 30 mg dose. Use the lowest effective dose needed to maintain response
- For 65+ years: Recommended dosage is 15 mg once daily
Please see Important Safety Information, including BOXED WARNING on Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis, below.
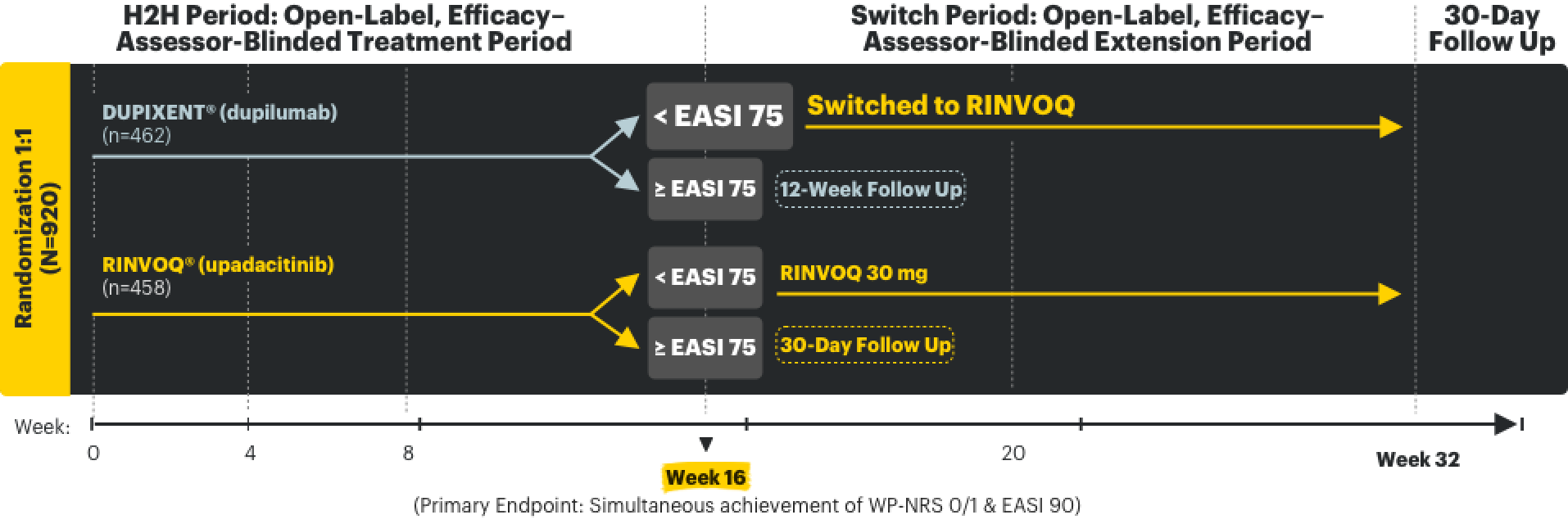
LEVEL UP Clinical Trial1,2,5
LEVEL UP: A prospective, open-label, assessor-blinded study in RINVOQ's indicated population with the approved starting dose
LEVEL UP was a phase 3b/4, multicenter, randomized, open-label, 2-part, head-to-head (H2H), efficacy–assessor-blinded study that evaluated the safety and efficacy of upadacitinib vs dupilumab in moderate to severe atopic dermatitis patients who were not adequately controlled with systemic therapy or when use of those therapies are inadvisable. After 16 weeks of the H2H Period, inadequate responders (<EASI 75) entered the Switch Period of the study, at which time those taking dupilumab were switched to upadacitinib and evaluated for an additional 16 weeks. In both study periods, upadacitinib was dosed as 15 mg once daily starting and was dose-adjusted to 30 mg based on clinical response, per protocol, starting at 4 weeks. In the H2H Period, dupilumab patients were dosed per its label.
Clinical decisions regarding treatment selection should take into account all relevant information, including full benefit-risk profiles in each product’s full Prescribing Information.
Conclusions comparing the overall benefit-risk profile of 2 active therapies cannot be made on a single clinical trial alone.

is indicated for adults and pediatric patients 12 years of age and older with refractory, moderate to severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies are inadvisable.
Boxed Warning on serious infections, mortality, malignancies, major adverse cardiovascular events, and thrombosis

is indicated for patients 6+ months of age with moderate to severe AD not adequately controlled with topical prescription therapies.
No Boxed Warning
These are not all the risks associated with RINVOQ and DUPIXENT. Please see additional Important Safety Information by clicking the Label Highlights here.
‡DUPIXENT is a registered trademark of Sanofi Biotechnology. See US Prescribing Information for more information.
HIGHER-LEVEL DISEASE CONTROL* in both itch & skin
*Defined by simultaneous achievement of WP-NRS 0/1 & EASI 90; higher than WP-NRS ≥4 improvement and EASI 75. Itch & skin are clinical features of AD; other outcome measures may exist.
While most studies focus on adequate control over itch and skin in AD, AbbVie is the first company to combine WP-NRS 0/1 & EASI 90 data as a primary endpoint in the Head-to-Head (H2H) Period of the LEVEL UP study—elevating what control could mean for AD.1-3,5,7,8*
The LEVEL UP study consisted of 2 periods2,9:
- In the H2H Period, patients were randomized to treatment with RINVOQ vs DUPIXENT for 16 weeks.
- In the Switch Period, patients switched treatment to RINVOQ for 16 weeks if they had an inadequate response§ to DUPIXENT in the H2H Period.
§Defined as patients with <EASI 75 response at Week 16 (end of H2H Period).


LEVEL UP H2H Period
Significantly more RINVOQ patients achieved higher-level disease control* vs DUPIXENT
at Week 162,6
Proportion of patients achieving itch and skin improvement RINVOQ vs DUPIXENT at Week 162,6
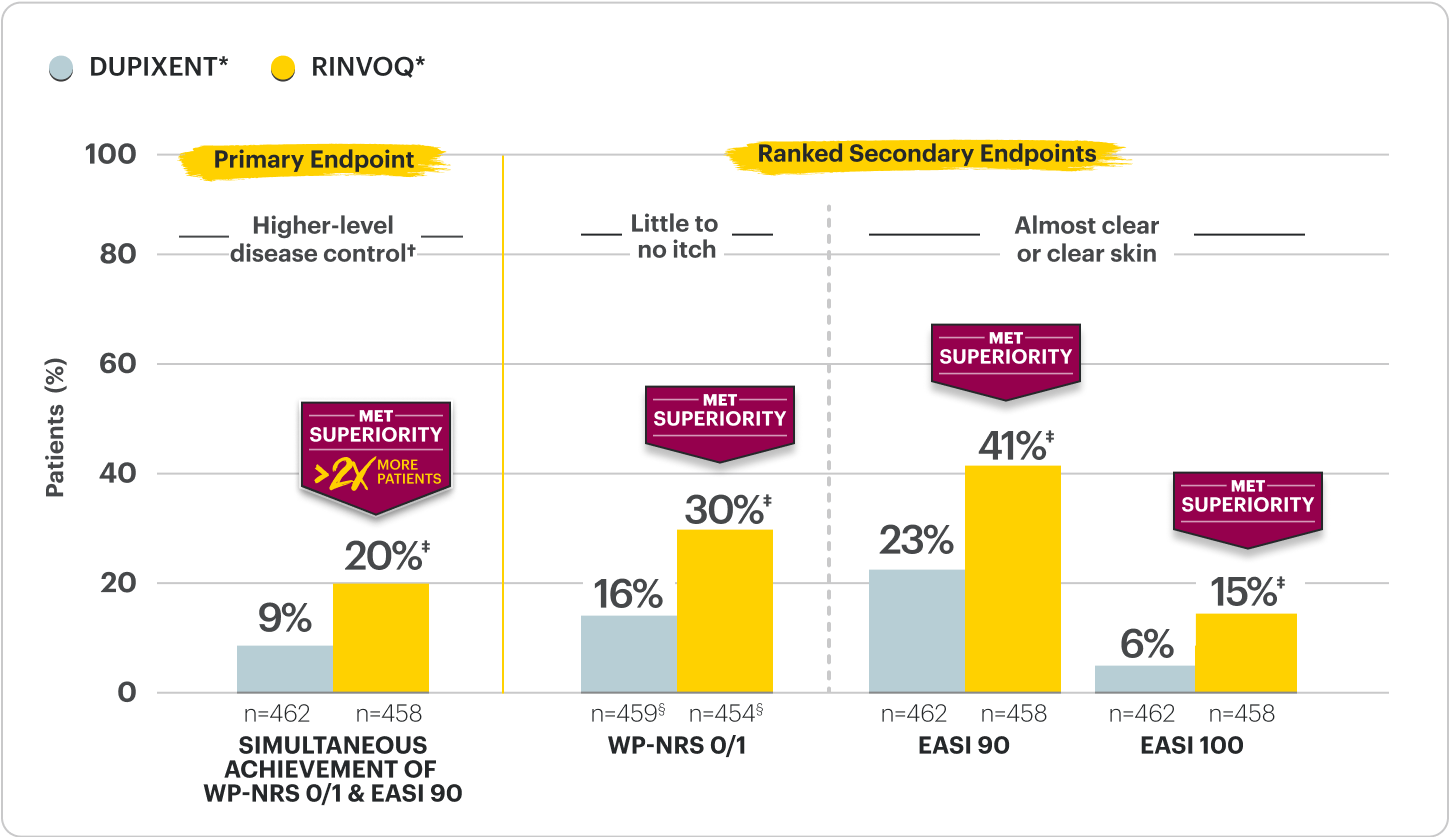
†RINVOQ was dosed as 15 mg once daily starting and dose-adjusted to 30 mg based on clinical response per protocol starting at Week 4 versus DUPIXENT, per its labeled dose. The study was powered to evaluate all RINVOQ- vs all DUPIXENT-treated patients without regard to dose.
*Defined by simultaneous achievement of WP-NRS 0/1 & EASI 90; higher than WP-NRS ≥4 improvement and EASI 75. Itch & skin are clinical features of AD; other outcome measures may exist.
‡p<0.0001; NRI-MI, ITT.
§n-value includes patients whose baseline WP-NRS is >1.
LEVEL UP is an open-label, phase 3b/4, multicenter, randomized, 2-part trial in moderate to severe atopic dermatitis patients not adequately controlled with systemic therapy or when use of those therapies are inadvisable. The H2H Period was a randomized, 16-week, efficacy-assessor-blinded study that evaluated the safety and assessor-blinded efficacy of upadacitinib vs dupilumab.
DATA LIMITATIONS:
Due to the open-label study design, patients knew the drug they were taking, which may have introduced bias.
RINVOQ and DUPIXENT have different indications as well as Warnings and Precautions (including BOXED WARNING for RINVOQ). Please refer to Label Highlights for differences in Safety Profiles.
Proportion of patients achieving itch (WP-NRS 0/1) and skin (EASI 90) improvement in the H2H Period
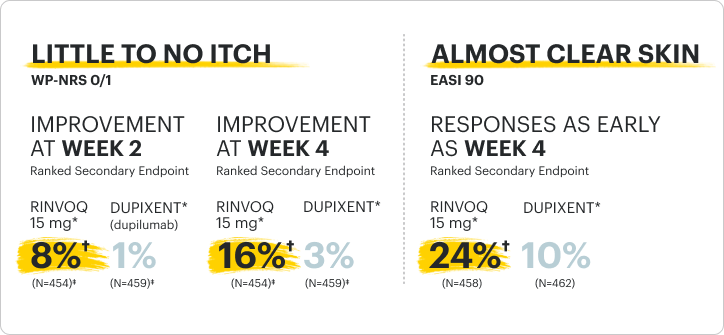
*RINVOQ was dosed as 15 mg once daily starting and dose-adjusted to 30 mg based on clinical response per protocol starting at Week 4 versus DUPIXENT, per its labeled dose. The study was powered to evaluate all RINVOQ- vs all DUPIXENT-treated patients without regard to dose.
†p<0.0001; NRI-MI, ITT.
‡n-value includes patients whose baseline WP-NRS is >1.
LEVEL UP is an open-label, phase 3b/4, multicenter, randomized, 2-part trial in moderate to severe atopic dermatitis patients not adequately controlled with systemic therapy or when use of those therapies are inadvisable. The H2H Period was a randomized, 16-week, efficacy-assessor-blinded study that evaluated the safety and assessor-blinded efficacy of upadacitinib vs dupilumab.
DATA LIMITATIONS:
Due to the open-label study design, patients knew the drug they were taking, which may have introduced bias.
RINVOQ and DUPIXENT have different indications as well as Warnings and Precautions (including BOXED WARNING for RINVOQ). Please refer to Label Highlights for differences in Safety Profiles.

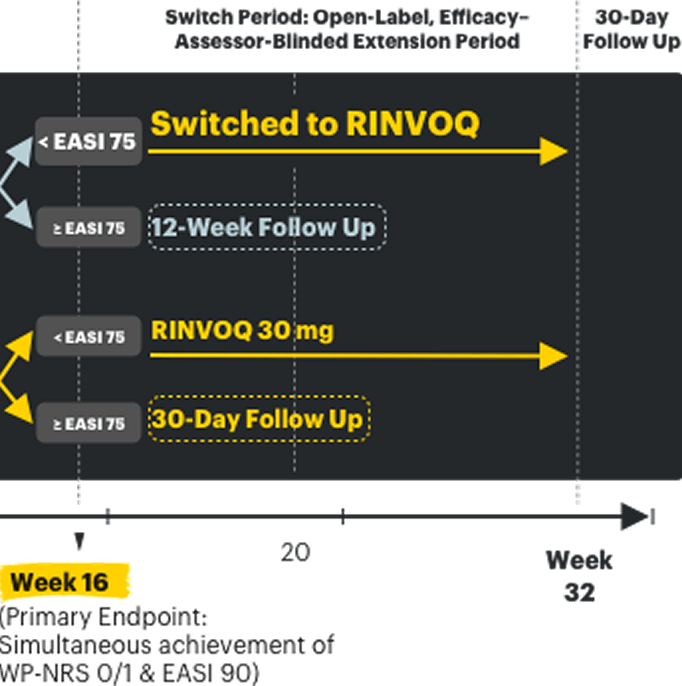
LEVEL UP Switch Period2,13
Objective of Switch Period: The 16-week Switch Period was designed to assess the safety and efficacy of RINVOQ* in DUPIXENT inadequate responders, who were defined as not having achieved ≥EASI 75 at Week 16 of the LEVEL UP H2H Period.
DUPIXENT inadequate responders entered the Switch Period (n=208) with:
- Mean skin clearance of ~EASI 50 (Mean EASI score of 14)
- Mean WP-NRS of 4.1
Mean itch and skin values reported for DUPIXENT inadequate responders are characteristics observed at entry of the Switch Period prior to initiation of RINVOQ. There was no treatment washout between study periods. All efficacy results from the Switch Period are observed cases and descriptive in nature.
*RINVOQ was dosed as 15 mg once daily starting and was dose-adjusted to 30 mg per protocol based on clinical response starting at Week 20 (4 weeks after switch), per its labeled dose. Data reported at Week 32 for all RINVOQ-treated patients were without regard to dose.
Proportion of patients on RINVOQ* achieving itch and skin improvements 16 weeks after switch2,3,14
Data are Observed Cases† and Descriptive in Nature
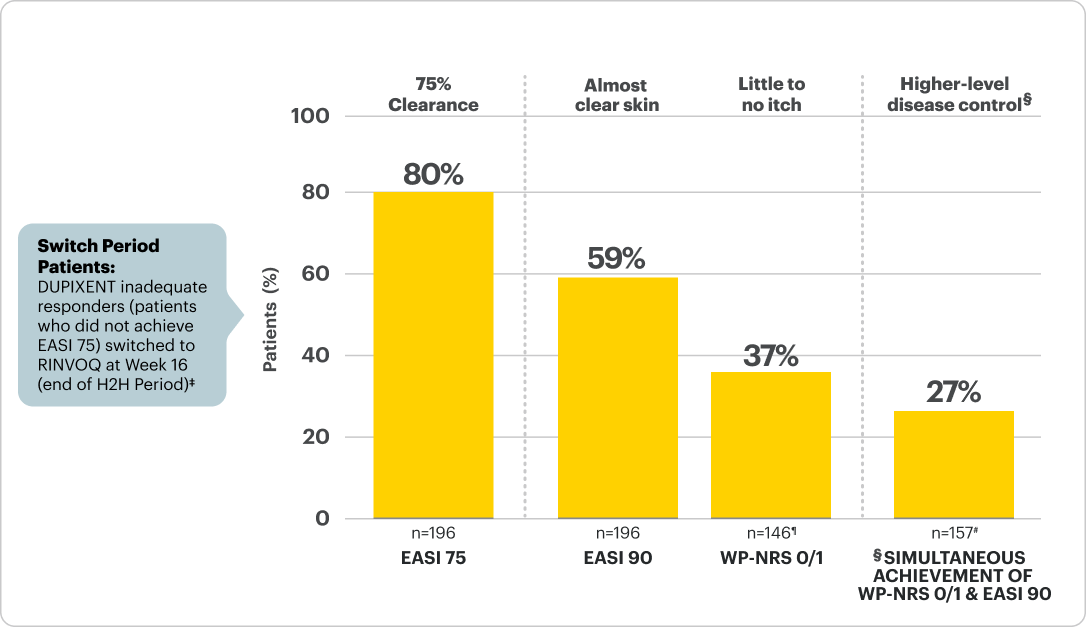
*RINVOQ was dosed as 15 mg once daily starting and was dose-adjusted to 30 mg per protocol based on clinical response starting at Week 20 (4 weeks after switch), per its labeled dose. Data reported at Week 32 for all RINVOQ-treated patients were without regard to dose.
†The open-label extension data shown here are a subanalysis and include only patients from LEVEL UP who were originally randomized to DUPIXENT, completed the H2H Period, and then enrolled in the Switch Period.
‡Itch & skin are clinical features of AD; other outcome measures may exist.
§n-value includes patients whose baseline WP-NRS >1 and did not achieve WP-NRS 0/1 at Week 16.
¶n-value includes patients who did not achieve WP-NRS 0/1 & EASI 90 at Week 16.
Patients who initiated topical rescue in the Switch Period following start of RINVOQ treatment were imputed as non-responders.
LEVEL UP is an open-label, phase 3b/4, multicenter, 2-part trial in moderate to severe atopic dermatitis patients not adequately controlled with systemic therapy or when use of those therapies are inadvisable. The Switch Period was a 16-week, open-label, efficacy–assessor-blinded extension period for patients with a <EASI 75 response at the conclusion of H2H Period (Week 16). The study period occurred from Weeks 16-32.
DATA LIMITATIONS:
Due to the open-label study design, patients knew the drug they were taking, which may have introduced bias. All data shown are prespecified, non-ranked endpoints. No clinical conclusions can be drawn.
RINVOQ and DUPIXENT have different indications as well as Warnings and Precautions (including BOXED WARNING for RINVOQ). Please refer to Label Highlights for differences in Safety Profiles.
Proportion of patients on RINVOQ* achieving itch and skin improvements 4 weeks and 16 weeks after switch2,3,14
Data are Observed Cases† and Descriptive in Nature

*RINVOQ was dosed as 15 mg once daily starting and was dose-adjusted to 30 mg per protocol based on clinical response starting at Week 20 (4 weeks after switch), per its labeled dose. Data reported at Week 32 for all RINVOQ-treated patients were without regard to dose.
†The open-label extension data shown here are a subanalysis and include only patients from LEVEL UP who were originally randomized to DUPIXENT, completed H2H Period, and then enrolled in Switch Period.
‡Itch & skin are clinical features of AD; other outcome measures may exist.
§n-value includes patients whose baseline WP-NRS >1 and did not achieve WP-NRS 0/1 at Week 16.
¶n-value includes patients who did not achieve WP-NRS 0/1 & EASI 90 at Week 16.
Patients who initiated topical rescue in Switch Period following start of RINVOQ treatment were imputed as non-responders.
LEVEL UP is an open-label, phase 3b/4, multicenter, 2-part trial in moderate to severe atopic dermatitis patients not adequately controlled with systemic therapy or when use of those therapies are inadvisable. The Switch Period was a 16-week, open-label, efficacy–assessor-blinded extension period for patients with a <EASI 75 response at the conclusion of H2H Period (Week 16). The study period occurred from Weeks 16-32.
DATA LIMITATIONS:
Due to the open-label study design, patients knew the drug they were taking, which may have introduced bias. All data shown are prespecified, non-ranked endpoints. No clinical conclusions can be drawn.
RINVOQ and DUPIXENT have different indications as well as Warnings and Precautions (including BOXED WARNING for RINVOQ). Please refer to Label Highlights for differences in Safety Profiles.
Safety Considerations
Serious Infections: RINVOQ-treated patients are at increased risk of serious bacterial (including tuberculosis [TB]), fungal, viral, and opportunistic infections leading to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants, such as methotrexate or corticosteroids.
Mortality: A higher rate of all-cause mortality, including sudden cardiovascular (CV) death, was observed with a Janus kinase inhibitor (JAKi) in a study comparing another JAKi with tumor necrosis factor (TNF) blockers in rheumatoid arthritis (RA) patients ≥50 years with ≥1 CV risk factor.
Malignancies: Malignancies have occurred in RINVOQ-treated patients. A higher rate of lymphomas and lung cancer (in current or past smokers) was observed with another JAKi when compared with TNF blockers in RA patients.
Major Adverse Cardiovascular Events: A higher rate of CV death, myocardial infarction, and stroke was observed with a JAKi in a study comparing another JAKi with TNF blockers in RA patients ≥50 years with ≥1 CV risk factor. History of smoking increases risk.
Thromboses: Deep venous thrombosis, pulmonary embolism, and arterial thrombosis have occurred in patients treated for inflammatory conditions with JAK inhibitors, including RINVOQ. A higher rate of thrombosis was observed with another JAKi when compared with TNF blockers in RA patients.
Hypersensitivity: RINVOQ is contraindicated in patients with hypersensitivity to RINVOQ or its excipients.
Other Serious Adverse Reactions: Hypersensitivity Reactions, Gastrointestinal Perforations, Laboratory Abnormalities, and Embryo-Fetal Toxicity.
Adverse Events (AEs) of Special Interest at Week 16 in Pivotal Trials and LEVEL UP H2H Period, and 16 Weeks After Switch in LEVEL UP Switch Period
AEs of special interest were generally consistent across multiple RINVOQ AD studies
<<Swipe table to see more
| All Subjects MEASURE UP 1, 2, AD UP, and Phase 2b |
All Subjects LEVEL UP H2H Period |
DUPIXENT-IR* patients LEVEL UP Switch Period |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (N=902) (PY=255.0) % (n/100 PY) | RINVOQ 15 mg (N=899) (PY=271.2) % (n/100 PY) | RINVOQ 30 mg (N=906) (PY=273.3) % (n/100PY) | RINVOQa (N=458) (PY=137.3) % (n/100 PY) | DUPIXENTa (N=461) (PY=139.4) % (n/100 PY) |
Patients switched to RINVOQa (N=208) (PY=63.1) % (n/100 PY) |
|||||||||
| INFECTIONS | ||||||||||||||
| Any serious infection | 0.6 (2.0) | 0.8 (2.6) | 0.4 (1.5) | 0 | 0.2 (0.7) | 0 | ||||||||
| Active TB | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| Opportunistic infection (excluding TB and herpes zoster)b |
0.4 (1.6) | 0.7 (2.2) | 0.8 (2.6) | 1.1 (3.7) | 0 | 0.5 (1.6) | ||||||||
| Herpes zoster | 0.6 (2.0) | 1.6 (5.2) | 1.5 (5.2) | 1.7 (5.9) | 0.4 (1.4) | 1 (3.2) | ||||||||
| MORTALITY | ||||||||||||||
| Mortality | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| MALIGNANCY | ||||||||||||||
| Malignancy (excluding NMSC)c | 0 | 0 | 0.4 (1.5) | 0 | 0 | 0 | ||||||||
| NMSCc | 0 | 0.3 (1.1) | 0.2 (0.7) | 0 | 0 | 0 | ||||||||
| CARDIOVASCULAR EVENTS | ||||||||||||||
| Adjudicated MACEc | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| Adjudicated VTEc | 0.1 (0.4) | 0 | 0 | 0 | 0 | 0 | ||||||||
| GASTROENTEROLOGICAL EVENTS | ||||||||||||||
| Adjudicated GI perforationsc | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
LEVEL UP was not designed or statistically powered to evaluate safety outcomes, including between RINVOQ and DUPIXENT nor to detect the risks revealed by the larger and longer XELJANZ ORAL Surveillance post-marketing study.
RINVOQ has a BOXED WARNING which includes Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis.
Safety rates in the LEVEL UP study were generally consistent with the known profile of RINVOQ for atopic dermatitis.
*Defined as patients with <EASI 75 response at Week 16 (end of H2H Period).
aIn the H2H Period, RINVOQ was dosed as 15 mg once daily starting and dose-adjusted to 30 mg based on clinical response, per protocol, starting at Week 4 versus DUPIXENT, per its labeled dose. In the Switch Period, RINVOQ was dosed as 15 mg once daily starting and dose-adjusted to 30 mg based on clinical response, per protocol, starting at Week 20 (4 weeks after switch) in the cohort switching from DUPIXENT.
See full Prescribing Information for DUPIXENT, which reflects different rates of AEs from those observed in the LEVEL UP clinical trial.5
bAll events were eczema herpeticum.
cPer protocol, patients experiencing AEs of malignancy (except for localized NMSC or cancer of the cervix in situ), adjudicated GI perforations, and adjudicated VTE were required to discontinue therapy.
AEs of special interest: AEs with an onset date that is on or after the first dose of study drug and no more than 30 days after the last dose of upadacitinib and placebo.
IR=inadequate responder; MACE=major adverse cardiovascular event; n/100 PY=number of subjects with at least 1 event per 100 PY; NMSC=nonmelanoma skin cancer; PY=patient-years; TB=tuberculosis; VTE=venous thromboembolism.
<<Swipe table to see more
| RINVOQ® (upadacitinib) | DUPIXENT® (dupilumab) | ||
|---|---|---|---|
| Indication and Usage | Adults and pediatric patients 12 years of age and older with refractory, moderate to severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies are inadvisable. | Adult and pediatric patients aged 6 months and older with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. DUPIXENT can be used with or without topical corticosteroids. | |
| Limitations of Use | RINVOQ is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants. | None | |
| Boxed Warnings |
|
None | |
| Contraindications | Known hypersensitivity to upadacitinib or any of the excipients in RINVOQ | Known hypersensitivity to dupilumab or any excipients in DUPIXENT. | |
| Warnings and Precautions |
|
|
|
| Adverse Reactions | Atopic Dermatitis: Adverse reactions (≥1%) are: upper respiratory tract infections, acne, herpes simplex, headache, blood creatine phosphokinase increased, cough, hypersensitivity, folliculitis, nausea, abdominal pain, pyrexia, increased weight, herpes zoster, influenza, fatigue, neutropenia, myalgia, and influenza like illness. |
Atopic Dermatitis (incidence ≥1%): Injection site reactions, conjunctivitis, blepharitis, oral herpes, keratitis, eye pruritus, other herpes simplex virus infection, dry eye, and eosinophilia. |
|
| Drug Interactions |
|
None | |
| Use in Specific Populations |
|
None |
Please see additional Important Safety Information for RINVOQ.
Please see Full Prescribing Information for RINVOQ.