WELL-STUDIED
SAFETY
PROFILE
INDICATION
RINVOQ is indicated for the treatment of adults and pediatric patients 12 years of age and older with refractory, moderate to severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies are inadvisable.
Limitations of Use: RINVOQ is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.
12 YEARS of clinical trial experience across 9 indications1-5*

27 CLINICAL TRIALS establishing a breadth of experience across indications

>15,000
patients in global clinical trials across US-approved indications
~6 YEARSof real-world experience1

APPROVED IN 9 INDICATIONS
RA, GCA, AD, UC, AS, nr-axSpA, CD, and pediatric patients 2+ years in pJIA† and PsA‡

Over 459,000 patients
prescribed globally across indications since 20191,6§
*Clinical experience encompasses the time from first RINVOQ patient dosed in RA clinical trial to present.
†pJIA: in pediatric TNFi-IR patients 2 years of age and older with active polyarticular juvenile idiopathic arthritis.
‡PsA: in adults and pediatric TNFi-IR patients 2 years of age and older with active psoriatic arthritis.
§Based on prescription data with RINVOQ in patients with RA, PsA, nr-axSpA, AS, AD, UC, or CD as of December 2024.6
AD=atopic dermatitis; AS=ankylosing spondylitis; CD=Crohn’s disease; GCA=giant cell arteritis; JAK=Janus kinase; nr-axSpA=non-radiographic axial spondyloarthritis; pJIA=polyarticular juvenile idiopathic arthritis; PsA=psoriatic arthritis; RA=rheumatoid arthritis; UC=ulcerative colitis.
Please see Important Safety Information, including BOXED WARNING on Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis, below.
Safety Data at Week 16
Adverse Events (AEs) of Special Interest in All Subjects Through Week 16: Integrated Safety7
All Subjects: Week 16
(MEASURE UP 1 and 2, AD UP, and Phase 2b)
<<Swipe table to see more
| Placebo (N=902) (PY=255.0) % (n/100 PY) | RINVOQ 15 mg (N=899) (PY=271.2) % (n/100 PY) | RINVOQ 30 mg(N=906) (PY=273.3) % (n/100 PY) | |||
|---|---|---|---|---|---|
| INFECTIONS | |||||
| Any serious infection | 0.6 (2.0) | 0.8 (2.6) | 0.4 (1.5) | ||
| Active TB | 0 | 0 | 0 | ||
| Opportunistic infection (excluding TB and herpes zoster)a | 0.4 (1.6) | 0.7 (2.2) | 0.8 (2.6) | ||
| Herpes zoster | 0.6 (2.0) | 1.6 (5.2) | 1.5 (5.2) | ||
| MORTALITY | |||||
| Mortality | 0 | 0 | 0 | ||
| MALIGNANCY | |||||
| Malignancy (excluding NMSC)b | 0 | 0 | 0.4 (1.5) | ||
| NMSCb | 0 | 0.3 (1.1) | 0.2 (0.7) | ||
| CARDIOVASCULAR EVENTS | |||||
| Adjudicated MACEb | 0 | 0 | 0 | ||
| Adjudicated VTEb | 0.1 (0.4) | 0 | 0 | ||
| GASTROENTEROLOGICAL EVENTS | |||||
| Adjudicated GI perforationsb | 0 | 0 | 0 |
Adverse reaction rates observed in clinical trials may not fully characterize the risks of RINVOQ. Certain adverse events may require longer observation periods and longer-term patient exposure to ascertain risk.
The adverse reaction profile in the pediatric patients was similar to the adults.1
aAll events were eczema herpeticum.8
bPer protocol, patients experiencing AEs of malignancy (except for localized NMSC or cancer of the cervix in situ), adjudicated GI perforations, and adjudicated VTE were required to discontinue therapy.
MACE=major adverse cardiovascular event; n/100 PY=number of subjects with at least 1 event per 100 PY; NMSC=nonmelanoma skin cancer; PY=patient-years; TB=tuberculosis; VTE=venous thromboembolism.
AEs of special interest: AEs with an onset date that is on or after the first dose of study drug and no more than 30 days after the last dose of upadacitinib and placebo.
MACE defined as cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke.
Safety Considerations
Consider the Benefits and Risks for the Individual Patient Prior to Initiating Therapy with RINVOQ
Consistent Safety Rates Up to 6 Years
Adverse Events of Special Interest in All Subjects: Long-Term Integrated Safety8
<<Swipe table to see more
All subjects |
||
|---|---|---|
| Data up to 6 years as of August 2024 | RINVOQ 15 mg (N=1,337) (PY=4,435.2) E/100 PY | RINVOQ 30 mg (N=1,346) (PY=4,752.5) E/100 PY |
| INFECTIONS | ||
| Any serious infection | 2.2 | 2.6 |
| Active TB | <0.1 | <0.1 |
| Opportunistic infection (excluding TB, herpes zoster, and oral candidiasis) | 1.6 | 2.0 |
| Herpes zoster | 3.2 | 5.2 |
| MORTALITY | ||
| Mortality | <0.1 | 0.1 |
| MALIGNANCY | ||
| Malignancy (excluding NMSC)a | 0.3 | 0.5 |
| NMSCa | 0.4 | 0.4 |
| CARDIOVASCULAR EVENTS | ||
| Adjudicated MACEa | 0.2 | <0.1 |
| Adjudicated VTEa | 0.1 | 0.1 |
| GASTROENTEROLOGICAL EVENTS | ||
| Adjudicated GI perforationsa | 0 | <0.1 |
Adverse reaction rates observed in clinical trials may not fully characterize the risks of RINVOQ. Certain adverse events may require longer observation periods and longer-term patient exposure to ascertain risk.
The adverse reaction profile in the pediatric patients was similar to the adults.1
Long Term: safety data through August 2024; total exposure 9,188 PY.9
aRates shown are n/100 PY=number of subjects with at least 1 event per 100 PY. Per protocol, patients experiencing AEs of malignancy (except for localized NMSC or cancer of the cervix in situ), adjudicated GI perforations, and adjudicated VTE were required to discontinue therapy.
AE=adverse event; E/100 PY=events per 100 patient-years; GI=gastrointestinal; MACE=major adverse cardiovascular event; NMSC=nonmelanoma skin cancer; PY=patient-year; TB=tuberculosis; VTE=venous thromboembolism.
Safety Rates in Pediatric Patients Up to 6 Years
Adverse Events of Special Interest in Pediatric Patients 12+ Years: Integrated Safety7-9
(MEASURE UP 1, MEASURE UP 2, and AD UP)
<<Swipe table to see more
| 16 weeks | Pediatrics 12+: Up to 52 weeksb | Pediatrics 12+: Up to 6 years | |||||
|---|---|---|---|---|---|---|---|
| Data up to 6 years as of August 2024 | Placebo (N=115) (PY=33.1) E/100 PY | RINVOQ 15 mg (N=167) (PY=191.7) E/100 PY | RINVOQ 30 mg (N=166) (PY=195.9) E/100 PY | RINVOQ 15 mg (N=264) (PY=864.5) E/100 PY | RINVOQ 30 mg (N=265) (PY=915.0) E/100 PY | ||
| INFECTIONS | |||||||
| Any serious infection | 6.0 | 3.1 | 2.6 | 2.0 | 1.2 | ||
| Active TB | 0 | 0 | 0 | 0 | 0 | ||
| Opportunistic infection (excluding TB, herpes zoster, and oral candidiasis) | 0 | 0.5 | 0.5 | 1.5 | 0.5 | ||
| Herpes zoster | 0 | 1.0 | 4.1 | 2.0 | 2.7 | ||
| MORTALITY | |||||||
| Mortality | 0 | 0 | 0 | 0 | 0 | ||
| MALIGNANCY | |||||||
| Malignancy (excluding NMSC)a | 0 | 0 | 0 | 0.1 | 0 | ||
| NMSCa | 0 | 0 | 0 | 0 | 0 | ||
| CARDIOVASCULAR EVENTS | |||||||
| Adjudicated MACEa | 0 | 0 | 0 | 0 | 0 | ||
| Adjudicated VTEa | 0 | 0 | 0 | 0 | 0 | ||
| GASTROENTEROLOGICAL EVENTS | |||||||
| Adjudicated Gl perforationsa | 0 | 0 | 0 | 0 | 0 | ||
Adverse reaction rates observed in clinical trials may not fully characterize the risks of RINVOQ. Certain adverse events may require longer observation periods and longer-term patient exposure to ascertain risk.
The adverse reaction profile in the pediatric patients was similar to the adults.1
aRates shown are n/100 PY=number of subjects with at least 1 event per 100 PY. Per protocol, patients experiencing AEs of malignancy (except for localized NMSC or cancer of the cervix in situ), adjudicated GI perforations, and adjudicated VTE were required to discontinue therapy.
bLong-term safety data through November 24, 2020 (64%-66% of patients had >1 year of exposure to RINVOQ; total exposure=2788 PY).9
AE=adverse event; E/100 PY=events per 100 patient-years; GI=gastrointestinal; MACE=major adverse cardiovascular event; NMSC=nonmelanoma skin cancer; PY=patient-year; TB=tuberculosis; VTE=venous thromboembolism.
Study Designs
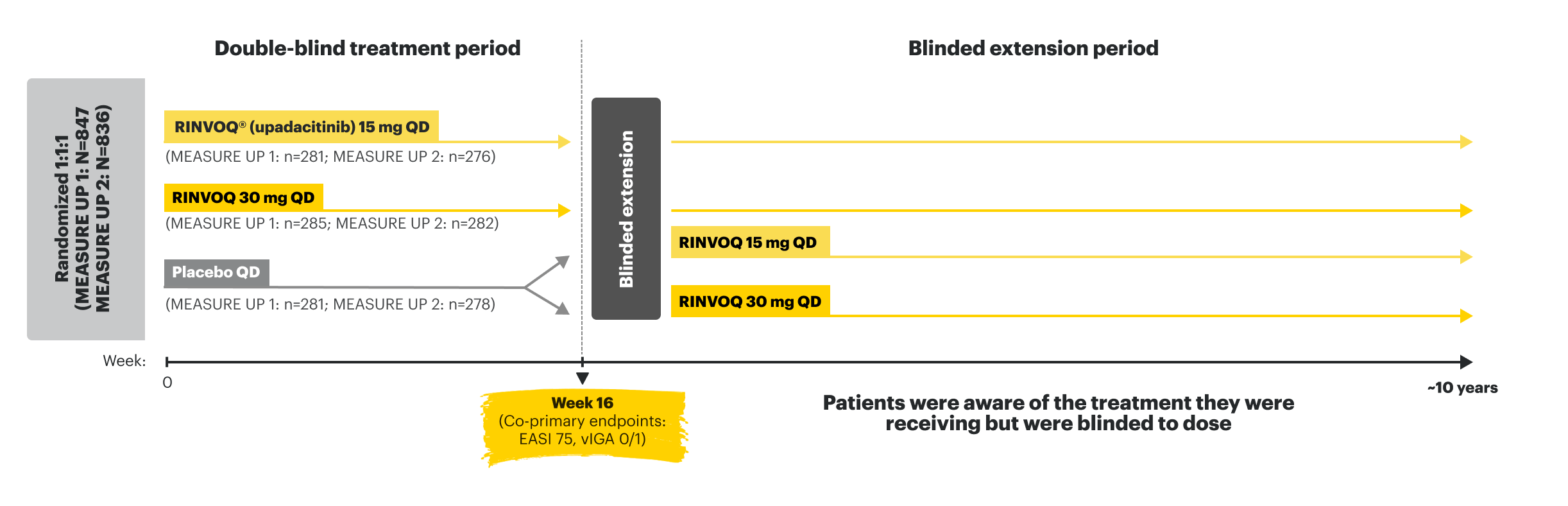
MEASURE UP 1 and 2
MEASURE UP 1 (N=847) and MEASURE UP 2 (N=836) were phase 3, multicenter, randomized, double-blind, parallel-group, placebo-controlled studies to evaluate the efficacy and safety of RINVOQ (15 mg or 30 mg) vs placebo over 16 weeks in adult and pediatric (≥12 years) patients with moderate to severe atopic dermatitis. Patients were randomized 1:1:1 to receive RINVOQ 15 mg, RINVOQ 30 mg, or placebo. Patients who completed the original 16-week, double-blind MEASURE UP studies entered the blinded extension treatment period. Following the completion of enrollment of MEASURE UP 1 and MEASURE UP 2, a supplemental study continued to enroll adolescent subjects until 180 adolescents were enrolled in MEASURE UP 1 and 180 adolescents were enrolled in MEASURE UP 2.1,10,11
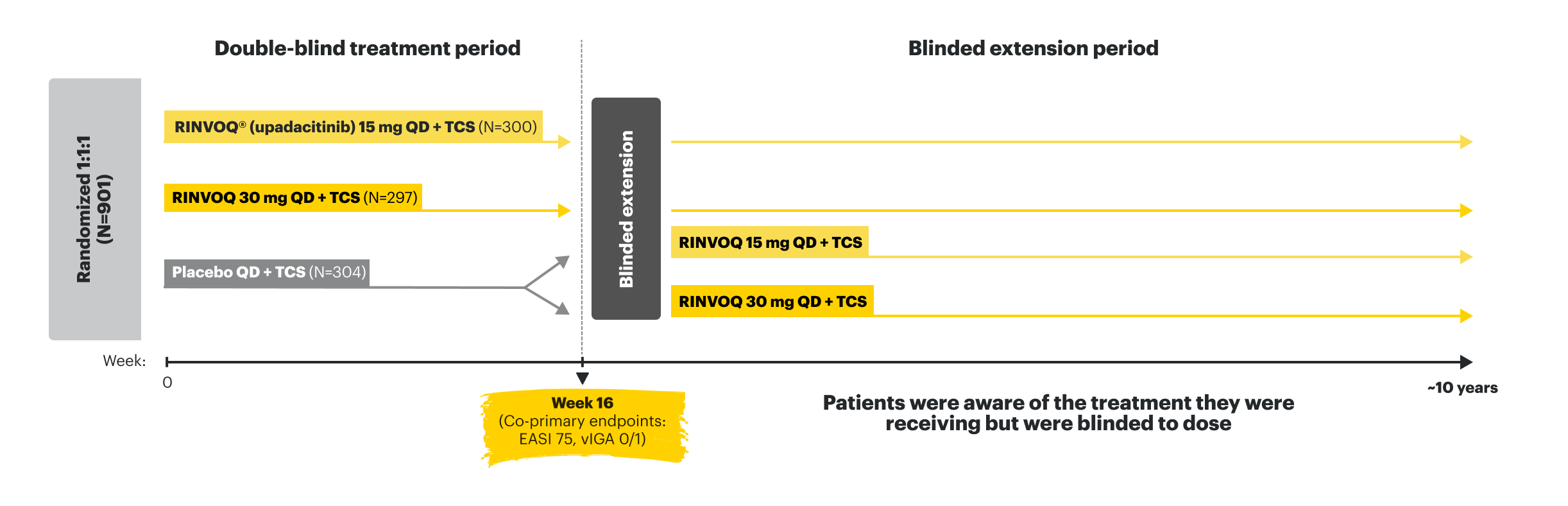
AD UP
AD UP (N=901) was a phase 3, multicenter, randomized, double-blind, parallel-group, placebo-controlled study to evaluate the efficacy and safety of RINVOQ (15 mg or 30 mg) + TCS vs placebo + TCS in adult and pediatric (≥12 years of age) patients with moderate to severe atopic dermatitis. Co-primary endpoints were EASI 75 and vIGA 0/1 response vs placebo at Week 16. Patients were randomized 1:1:1 to receive RINVOQ 15 mg, RINVOQ 30 mg, or placebo—all in combination with TCS. Patients who completed the original 16-week, double-blind AD UP study entered the blinded extension treatment period. Following the completion of enrollment in AD UP, a supplemental study continued to enroll adolescent subjects until 180 adolescents were enrolled in AD UP.11-13
Please see Important Safety Information, including BOXED WARNING on Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis, below.
Common Adverse Events
Most Common Adverse Events ≥1% in All Subjects Through Week 16: Integrated Safety1
<<Swipe table to see more
| All Subjects (MEASURE UP 1, MEASURE UP 2, AD UP, Phase 2b) |
|||||
|---|---|---|---|---|---|
| Placebo (n=902) % | RINVOQ 15 mg (n=899) % | RINVOQ 30 mg (n=906) % | |||
| Upper respiratory tract infectiona | 17 | 23 | 25 | ||
| Acneb | 2 | 10 | 16 | ||
| Herpes simplexc | 2 | 4 | 8 | ||
| Headache | 4 | 6 | 6 | ||
| Increased blood CPK | 2 | 5 | 6 | ||
| Cough | 1 | 3 | 3 | ||
| Hypersensitivityd | 2 | 2 | 3 | ||
| Folliculitis | 1 | 2 | 3 | ||
| Nausea | 1 | 3 | 3 | ||
| Abdominal paine | 1 | 3 | 2 | ||
| Pyrexia | 1 | 2 | 2 | ||
| Increased weight | 1 | 2 | 2 | ||
| Herpes zosterf | 1 | 2 | 2 | ||
| Influenza | <1 | 2 | 2 | ||
| Fatigue | 1 | 1 | 2 | ||
| Neutropenia | <1 | 1 | 2 | ||
| Myalgia | 1 | 1 | 2 | ||
| Influenza-like illness | 1 | 1 | 2 | ||
Adverse reaction rates observed in clinical trials and LTE studies may not predict the rates observed in a broader patient population in clinical practice.
aIncludes: laryngitis, laryngitis viral, nasopharyngitis, oropharyngeal pain, pharyngeal abscess, pharyngitis, pharyngitis streptococcal, pharyngotonsillitis, respiratory tract infection, respiratory tract infection viral, rhinitis, rhinolaryngitis, sinusitis, tonsillitis, tonsillitis bacterial, upper respiratory tract infection, viral pharyngitis, viral upper respiratory tract infection.
bIncludes: acne and dermatitis acneiform.
cIncludes: genital herpes, genital herpes simplex, herpes dermatitis, herpes ophthalmic, herpes simplex, nasal herpes, ophthalmic herpes simplex, herpes virus infection, oral herpes.
dIncludes anaphylactic reaction, anaphylactic shock, angioedema, dermatitis exfoliative generalized, drug hypersensitivity, eyelid oedema, face oedema, hypersensitivity, periorbital swelling, pharyngeal swelling, swelling face, toxic skin eruption, type I hypersensitivity, urticaria.
eIncludes abdominal pain and abdominal pain upper.
fIncludes herpes zoster and varicella.
CPK=creatine phosphokinase; LTE=long-term extension.
Most Common Adverse Events in ≥5% in Pediatrics 12+ Years Through Week 16: Integrated Safety7
<<Swipe table to see more
| Pediatrics (≥12 and <18 years of age) (MEASURE UP 1, MEASURE UP 2, AD UP, Phase 2b) |
|||||
|---|---|---|---|---|---|
| Placebo (n=115) % | RINVOQ 15 mg (n=114) % | RINVOQ 30 mg (n=114) % | |||
| Acne | 1 | 13 | 15 | ||
| Upper respiratory tract infection | 4 | 11 | 12 | ||
| Headache | 5 | 6 | 9 | ||
| Increased CPK | 3 | 5 | 8 | ||
| Nasopharyngitis | 5 | 10 | 7 | ||
| Vomiting | 1 | 1 | 6 | ||
| Diarrhea | 3 | 2 | 5 | ||
| Pyrexia | 0 | 3 | 5 | ||
| Influenza | 0 | 2 | 5 | ||
| Oropharyngeal pain | 0 | 5 | 2 | ||
| Dermatitis atopic | 10 | 4 | 2 | ||
Adverse reaction rates observed in clinical trials and LTE studies may not predict the rates observed in a broader patient population in clinical practice.
The adverse reaction profile in the pediatric patients was similar to the adults.1
Select Baseline Medical History in RINVOQ AD Phase 3 Clinical Trials
<<Swipe table to see more
| SELECT INTEGRATED BASELINE CHARACTERISTICS FROM MEASURE UP 1, MEASURE UP 2, AND AD UP1,8 | |||
|---|---|---|---|
| RINVOQ 15 mg (N=1,337) n (%) |
RINVOQ 30 mg (N=1,346) n (%) |
||
| Presence of CV risk factora | 723 (54.1) | 690 (51.3) | |
| History of tobacco/nicotine use | 418 (31.3) | 419 (31.1) | |
| History of hypertension | 149 (11.1) | 127 (9.4) | |
| History of CV event | 72 (5.4) | 74 (5.5) | |
| Diabetes mellitus | 27 (2.0) | 28 (2.1) | |
| Elevated LDL-C (≥130 mg/dL) | 199 (14.9) | 191 (14.2) | |
| Low HDL-C (<40 mg/dL) | 177 (13.2) | 168 (12.5) | |
| Age ≥50 with ≥1 CV risk factora | 179 (13.4) | 209 (15.5) | |
| Number of CV risk factorsa | |||
| 0 | 614 (45.9) | 656 (48.7) | |
| 1 | 484 (36.2) | 451 (33.5) | |
| 2 | 175 (13.1) | 176 (13.1) | |
| 3+ | 64 (4.7) | 63 (4.7) | |
| Obesity (BMI ≥30 kg/m2) | 252 (18.9) | 258 (19.3) | |
| History of VTE | 4 (0.3) | 6 (0.4) | |
| Oral contraceptive use | 105 (17.7) n=593 |
130 (22.2) n=585 |
|
aCV risk factors included CV event, hypertension, diabetes mellitus, tobacco/nicotine use, elevated LDL-C, and lowered HDL-C.
BMI=body mass index; CV=cardiovascular; HDL-C=high-density lipoprotein cholesterol; LDL-C=low-density lipoprotein cholesterol; OCP=oral contraceptives; VTE=venous thromboembolic event.
RINVOQ Was Studied in Patients 12-75 Years Old with Various Comorbidities AND CONTINUES TO BE STUDIED IN THESE PATIENTS IN ONGOING TRIALS8
>50% had at least 1 CV risk factora
>30% were current or former smokers
~20% of females were using OCPs
~15% were ≥50 years of age with ≥1 CV risk factora
Consider the benefits and risks for individual patients prior to initiating or continuing therapy with RINVOQ, particularly in those who are current or past smokers and patients with other CV risk factors. Discontinue RINVOQ in patients that have experienced myocardial infarction or stroke. Patients with symptoms of thrombosis should discontinue RINVOQ and be promptly evaluated. Avoid RINVOQ in patients that may be at increased risk for thrombosis.1
Additional Acne Considerations1,7
Through Week 16 in all subjects, acne events occurred in 10% and 16% of patients in the RINVOQ 15 mg group (n=899) and RINVOQ 30 mg group (n=906), respectively, compared with 2% in the placebo group (n=902).