
RA patients met ACR20 at Week 12 or 14 (primary endpoints) and disease control through remission (DAS28-CRP <2.6)* at Weeks 12 or 14 and observed up to 5 years.1-5
RA patients met ACR20 at Week 12 or 14 (primary endpoints) and disease control through remission (DAS28-CRP <2.6)* at Weeks 12 or 14 and observed up to 5 years.1-5
*Clinical remission does not mean drug-free remission or complete absence of disease activity.
ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; CRP=C-reactive protein; DAS28-CRP=28-joint disease activity score using C-reactive protein.
aRINVOQ is on a preferred tier or otherwise has preferred status on the plan’s formulary.
bCoverage requirements and benefit designs vary by payer and may change over time. Please consult with payers directly for the most current reimbursement policies.
INDICATION
RINVOQ is indicated for the treatment of adults with moderately to severely active rheumatoid arthritis (RA) who have had an inadequate response or intolerance to one or more tumor necrosis factor (TNF) blockers.
Limitations of Use: RINVOQ is not recommended for use in combination with other Janus kinase (JAK) inhibitors, biologic disease-modifying antirheumatic drugs (bDMARDs), or with potent immunosuppressants such as azathioprine and cyclosporine.
Exceptional Patient and Access Support
- ~99% preferred combined national commercial and Medicare Part D formulary coverage under the pharmacy benefit as of May 2025 in RA6,‡,§
- 1:1 support to help RA patients start and stay on track with their prescribed treatment plan
- Get patients started on RINVOQ Complete by downloading the enrollment form
*P<0.001. Analyses were not controlled for multiplicity. P-values obtained through nominal statistical testing.5
†In PsA: ~6.4 years maximum exposure (~3.6 years median) to RINVOQ 15 mg as of 08/2024. In AS: ~3.8 years maximum exposure (~1.8 years median) to RINVOQ 15 mg as of 08/2024. In nr-axSpA: ~2.3 years maximum exposure (~1.0 year median) to RINVOQ 15 mg as of 08/2024.7
‡RINVOQ is on a preferred tier or otherwise has preferred status on the plan's formulary.
§Coverage requirements and benefit designs vary by payer and may change over time. Please consult with payers directly for the most current reimbursement policies.
AS=ankylosing spondylitis; bDMARD=biologic disease-modifying antirheumatic drug; csDMARD=conventional synthetic disease-modifying antirheumatic drug; IR=intolerance or inadequate response; max.=maximum; MTX=methotrexate; nr-axSpA=non-radiographic axial spondyloarthritis; PBO=placebo; PsA=psoriatic arthritis; RA=rheumatoid arthritis;
Please see Important Safety Information, including BOXED WARNING on Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis, below.
RINVOQ (upadacitinib) Met All Primary and Ranked Secondary Endpoints1,8
Primary Endpoint Results (Week 12 or 14)
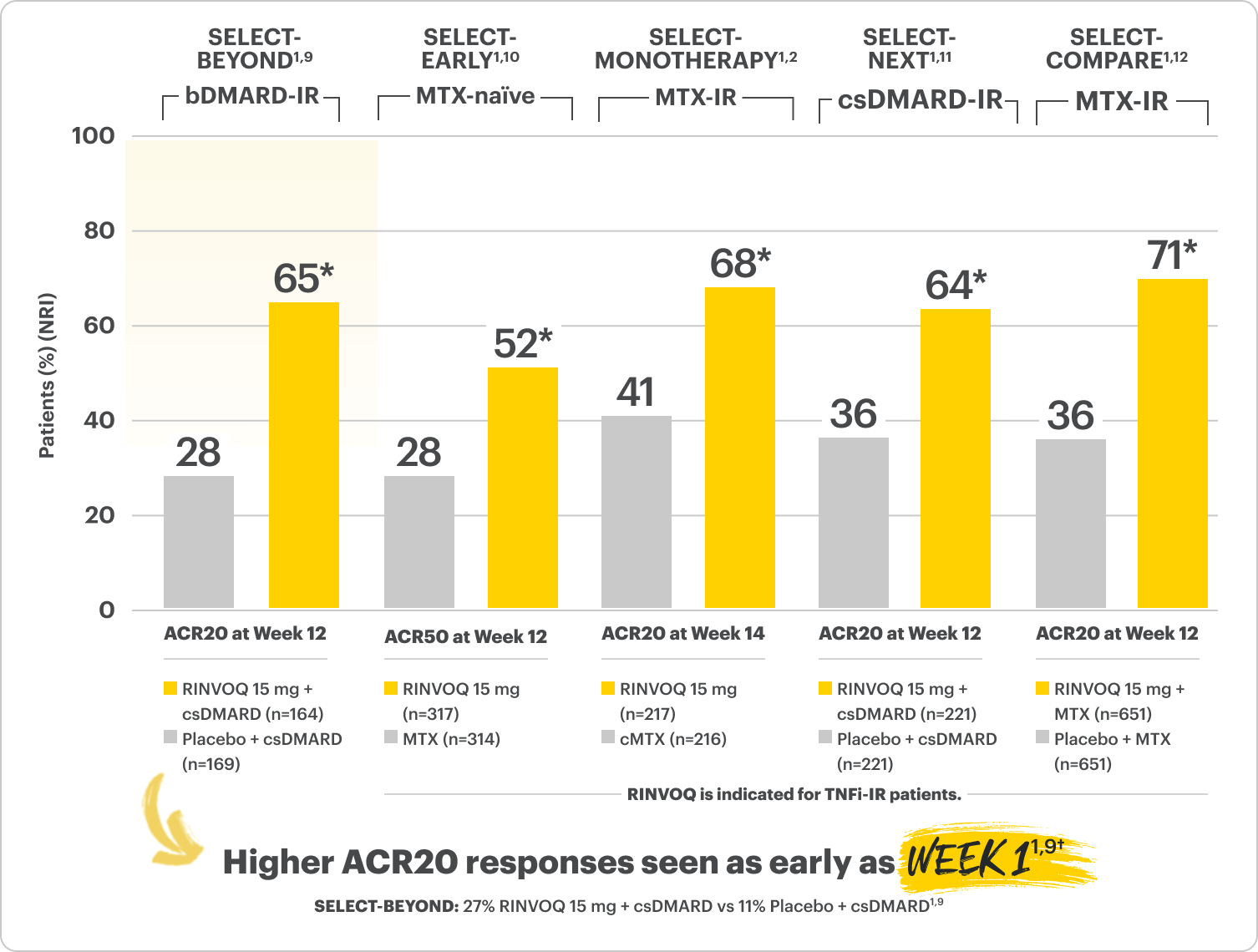
*P≤0.001 RINVOQ vs Placebo or MTX, †P=0.0001; Week 1 analyses were not controlled for multiplicity; P‑values obtained through nominal statistical testing.
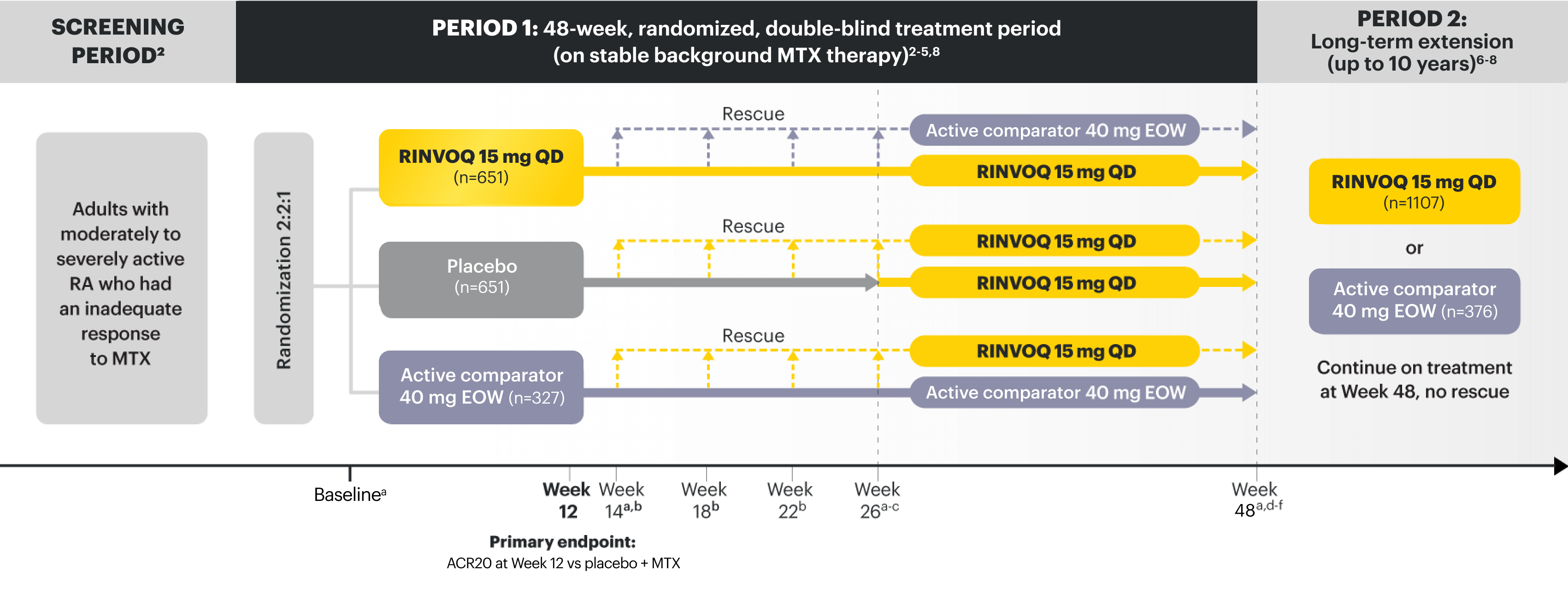
SELECT-BEYOND (STUDY RA-V):1,9 24-week, randomized, double-blind, placebo-controlled study of 499 adult patients with moderate to severe RA who had an inadequate response or intolerance to bDMARDs.
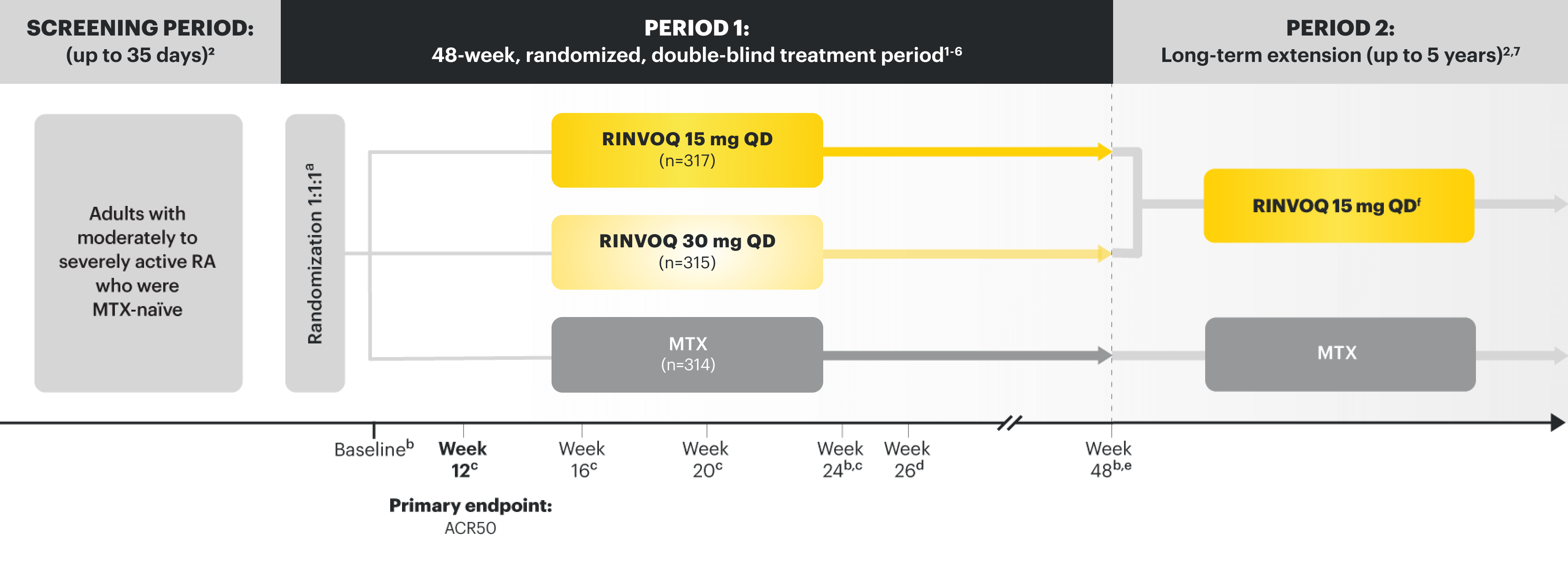
SELECT-EARLY (STUDY RA-I):1,10 48-week, randomized, double-blind, active comparator-controlled study of 947 adult patients with moderate to severe RA who were MTX naïve.
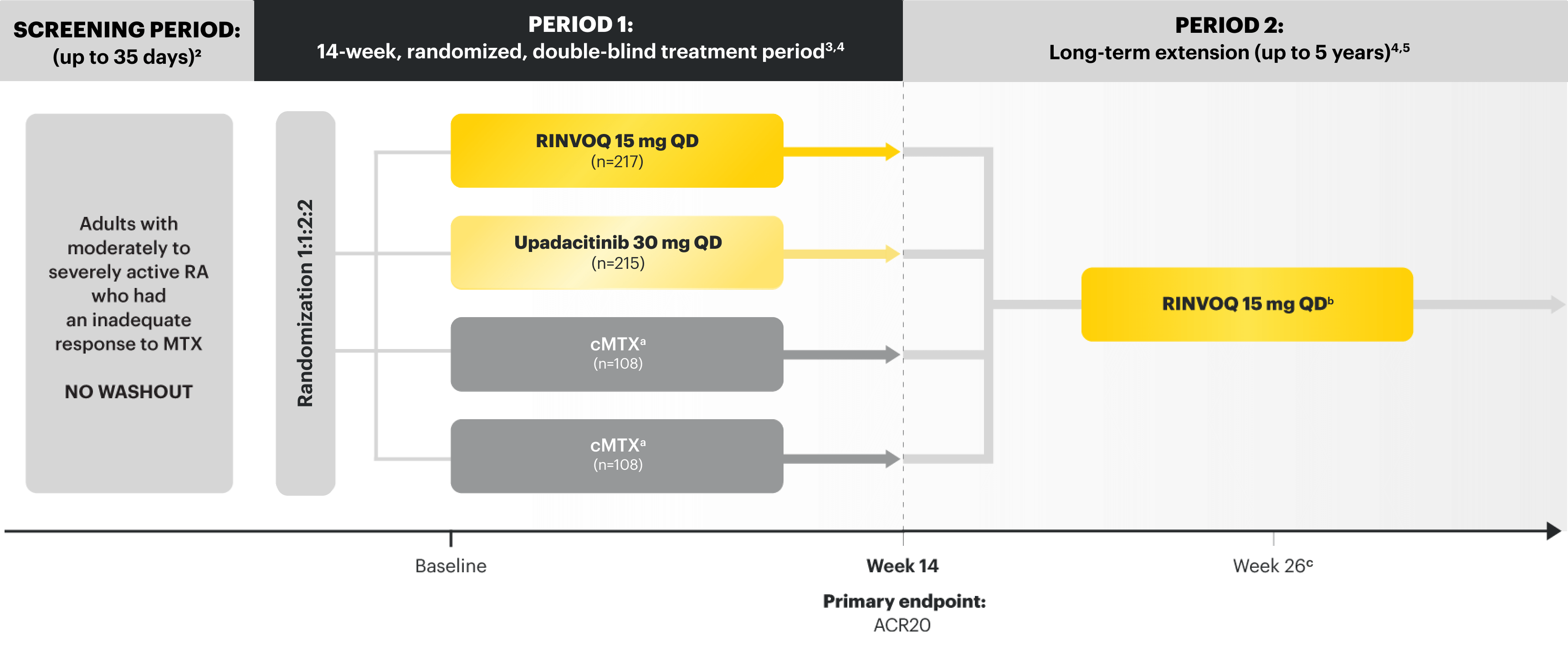
SELECT-MONOTHERAPY (STUDY RA-II):1,2 14-week, randomized, double-blind, active comparator-controlled study of 648 adult patients with moderate to severe RA who had an inadequate response to MTX.
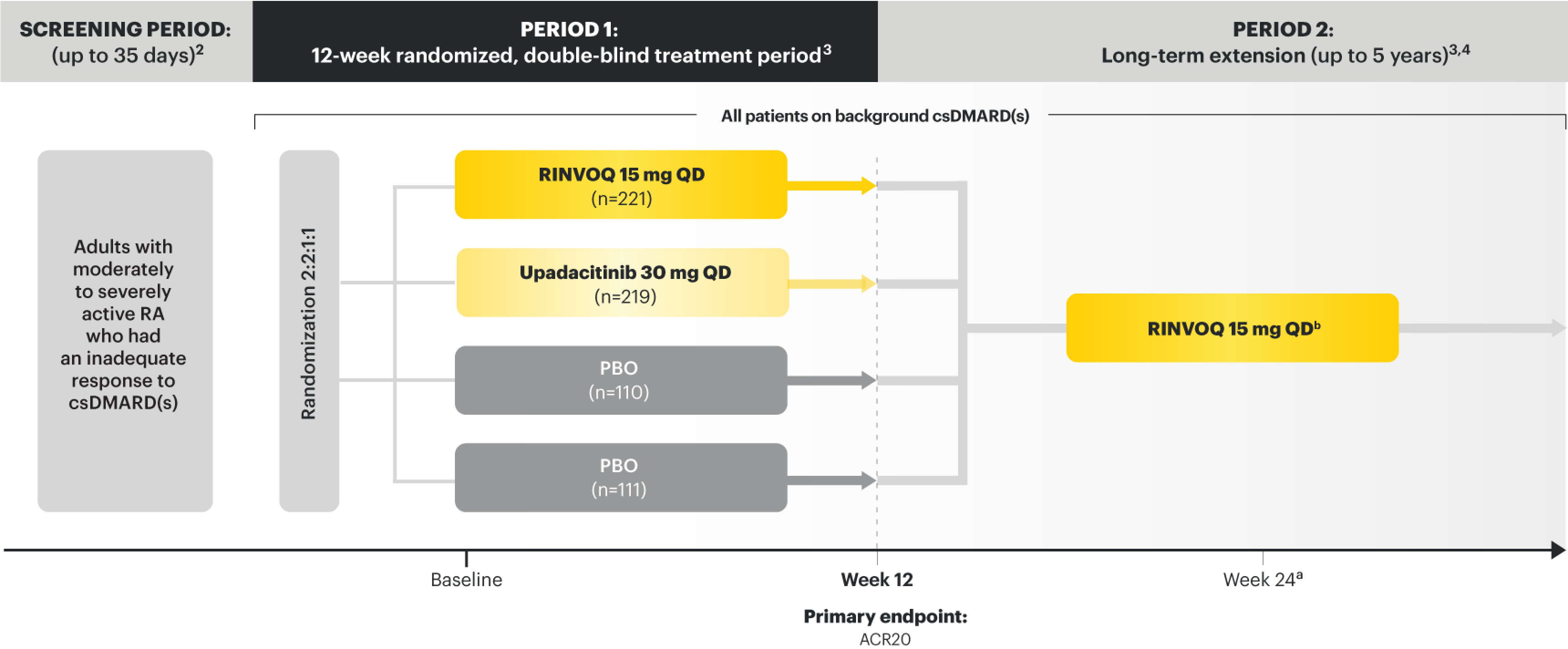
SELECT-NEXT (STUDY RA-III):1,11 12-week, randomized, double-blind, placebo-controlled study of 661 adult patients with moderate to severe RA who had an inadequate response to csDMARDs.
SELECT-COMPARE (STUDY RA-IV):1,12 48-week, randomized, double-blind, active comparator-controlled study of 1629 adult patients with moderate to severe RA who had an inadequate response to MTX.
ACR=American College of Rheumatology; bDMARD=biologic disease-modifying antirheumatic drug; cMTX=continued methotrexate; csDMARD=conventional synthetic disease-modifying antirheumatic drug; IR=intolerance or inadequate response; MTX=methotrexate; NRI=nonresponder imputation; RA=rheumatoid arthritis; TNFi=tumor necrosis factor inhibitor.